Abstract
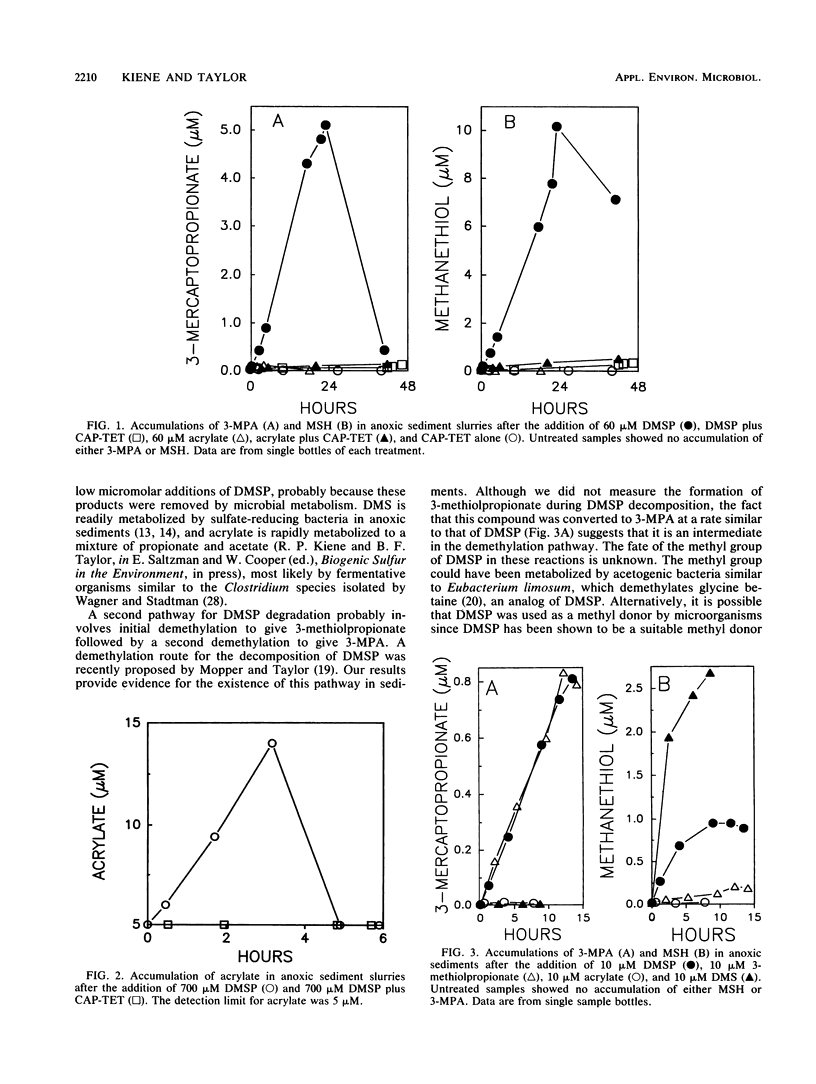
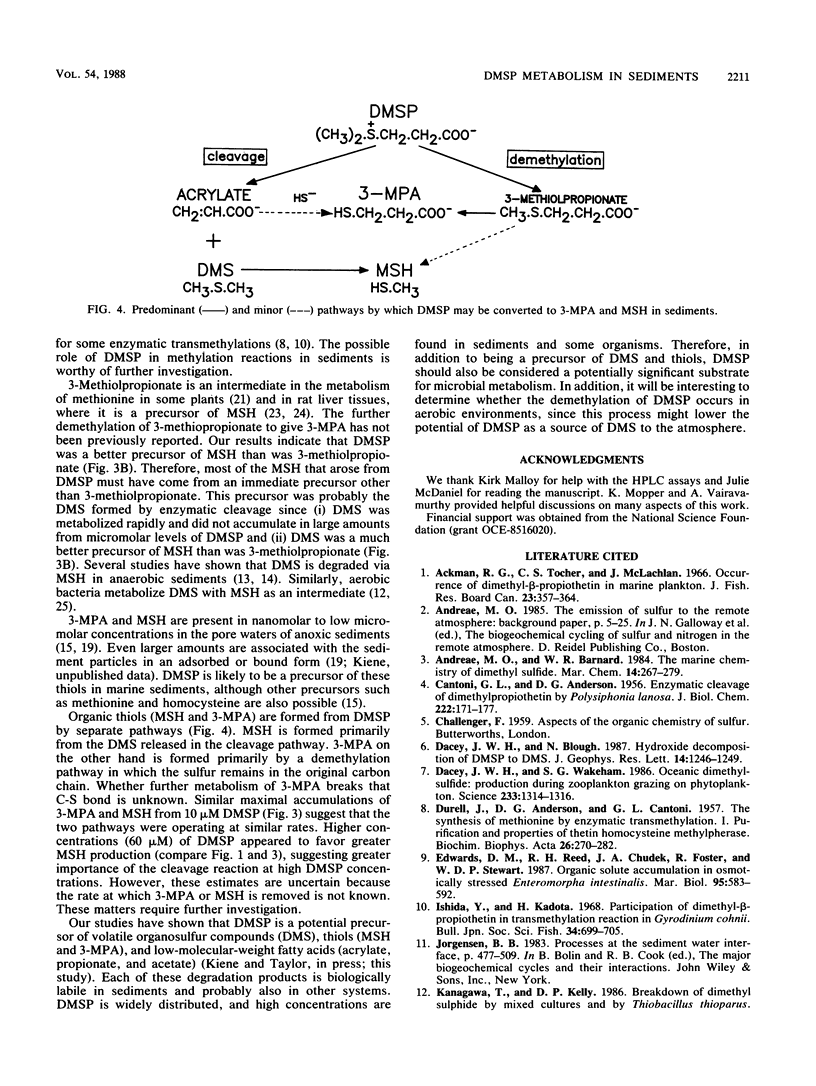
Dimethylsulfoniopropionate (DMSP) is a natural product of algae and aquatic plants, particularly those from saline environments. We investigated whether DMSP could serve as a precursor of thiols in anoxic coastal marine sediments. The addition of 10 or 60 μM DMSP to anoxic sediment slurries caused the concentrations of 3-mercaptopropionate (3-MPA) and methanethiol (MSH) to increase. Antibiotics prevented the appearance of these thiols, indicating biological formation. Dimethyl sulfide (DMS) and acrylate also accumulated after the addition of DMSP, but these compounds were rapidly metabolized by microbes and did not reach high levels. Acrylate and DMS were probably generated by the enzymatic cleavage of DMSP. MSH arose from the microbial metabolism of DMS, since the direct addition of DMS greatly increased MSH production. Additions of 3-methiolpropionate gave rise to 3-MPA at rates similar to those with DMSP, suggesting that sequential demethylation of DMSP leads to 3-MPA formation. Only small amounts of MSH were liberated from 3-methiolpropionate, indicating that demethiolation was not a major transformation for 3-methiolpropionate. We conclude that DMSP was degraded in anoxic sediments by two different pathways. One involved the well-known enzymatic cleavage to acrylate and DMS, with DMS subsequently serving as a precursor of MSH. In the other pathway, successive demethylations of the sulfur atom proceeded via 3-methiolpropionate to 3-MPA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON D. G., CANTONI G. L. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J Biol Chem. 1956 Sep;222(1):171–177. [PubMed] [Google Scholar]
- DURELL J., ANDERSON D. G., CANTONI G. L. The synthesis of methionine by enzymic transmethylation. I. Purification and properties of thetin homocysteine methylpherase. Biochim Biophys Acta. 1957 Nov;26(2):270–281. doi: 10.1016/0006-3002(57)90005-7. [DOI] [PubMed] [Google Scholar]
- Dacey J. W., Wakeham S. G. Oceanic dimethylsulfide: production during zooplankton grazing on phytoplankton. Science. 1986 Sep 19;233(4770):1314–1316. doi: 10.1126/science.233.4770.1314. [DOI] [PubMed] [Google Scholar]
- Kiene R. P., Oremland R. S., Catena A., Miller L. G., Capone D. G. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl Environ Microbiol. 1986 Nov;52(5):1037–1045. doi: 10.1128/aem.52.5.1037-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Visscher P. T. Production and fate of methylated sulfur compounds from methionine and dimethylsulfoniopropionate in anoxic salt marsh sediments. Appl Environ Microbiol. 1987 Oct;53(10):2426–2434. doi: 10.1128/aem.53.10.2426-2434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mopper K., Delmas D. Trace determination of biological thiols by liquid chromatography and precolumn fluorometric labeling with o-phthaladehyde. Anal Chem. 1984 Nov;56(13):2557–2560. doi: 10.1021/ac00277a064. [DOI] [PubMed] [Google Scholar]
- Müller E., Fahlbusch K., Walther R., Gottschalk G. Formation of N,N-Dimethylglycine, Acetic Acid, and Butyric Acid from Betaine by Eubacterium limosum. Appl Environ Microbiol. 1981 Sep;42(3):439–445. doi: 10.1128/aem.42.3.439-445.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. D., Benevenga N. J. Identification of 3-methylthiopropionic acid as an intermediate in mammalian methionine metabolism in vitro. J Biol Chem. 1978 Nov 10;253(21):7844–7850. [PubMed] [Google Scholar]
- Steele R. D., Benevenga N. J. The metabolism of 3-methylthiopropionate in rat liver homogenates. J Biol Chem. 1979 Sep 25;254(18):8885–8890. [PubMed] [Google Scholar]
- WAGNER C., STADTMAN E. R. Bacterial fermentation of dimethyl-beta-propiothetin. Arch Biochem Biophys. 1962 Aug;98:331–336. doi: 10.1016/0003-9861(62)90191-1. [DOI] [PubMed] [Google Scholar]


