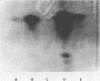
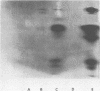
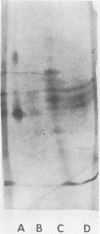
Abstract
The lignocellulose-degrading actinomycete Streptomyces viridosporus T7A produced an extracellular esterase when grown in a mineral salts-yeast extract medium. Extracellular esterase activity was first detected during the late stationary phase and typically followed the appearance of intracellular activity. When the organism was grown in lignocellulose-supplemented medium, esterase activity was not increased, but lignocellulose-esterified p-coumaric acid and vanillic acid were released into the medium. Polyacrylamide gels showed that several extracellular esterases differing in substrate specificity were produced. Ultrafiltration was used to concentrate the esterase prior to purification. Activity was recovered mostly in the molecular weight fraction between 10,000 and 100,000. Concentrated esterase was further purified by DEAE-Sepharose anion-exchange chromatography to a specific activity 11.82 times greater than that in the original supernatant. There were seven detectable esterase active proteins in the partially purified enzyme solution. Three were similar esterases that may be isoenzymes. The partially purified esterase had a pH optimum for activity of 9.0, a temperature optimum of 45 to 50°C, and a Km and Vmax of 0.030 mM and 0.097 μmol/min per ml, respectively, when p-nitrophenyl butyrate was the substrate. The enzyme was unstable above 40°C but retained activity when stored at 4 or −20°C. It lost some activity (20%) when lyophilized. Substrate specificity assays showed that it hydrolyzed ester linkages of p-nitrophenyl butyrate, α-naphthyl acetate, α-naphthyl butyrate, and lignocellulose. Vanillic and p-coumaric acids were identified as products released from lignocellulose. The enzyme is thought to be a component of the lignocellulose-degrading enzyme system of S. viridosporus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barder M. J., Crawford D. L. Effects of carbon and nitrogen supplementation on lignin and cellulose decomposition by a Streptomyces. Can J Microbiol. 1981 Aug;27(8):859–863. doi: 10.1139/m81-136. [DOI] [PubMed] [Google Scholar]
- Borgmeyer J. R., Crawford D. L. Production and Characterization of Polymeric Lignin Degradation Intermediates from Two Different Streptomyces spp. Appl Environ Microbiol. 1985 Feb;49(2):273–278. doi: 10.1128/aem.49.2.273-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Crawford D. L. Lignocellulose decomposition by selected streptomyces strains. Appl Environ Microbiol. 1978 Jun;35(6):1041–1045. doi: 10.1128/aem.35.6.1041-1045.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Pometto A. L., Crawford R. L. Lignin Degradation by Streptomyces viridosporus: Isolation and Characterization of a New Polymeric Lignin Degradation Intermediate. Appl Environ Microbiol. 1983 Mar;45(3):898–904. doi: 10.1128/aem.45.3.898-904.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackenzie C. R., Bilous D., Schneider H., Johnson K. G. Induction of Cellulolytic and Xylanolytic Enzyme Systems in Streptomyces spp. Appl Environ Microbiol. 1987 Dec;53(12):2835–2839. doi: 10.1128/aem.53.12.2835-2839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen D. A., Schottel J. L. Purification and characterization of a novel extracellular esterase from pathogenic Streptomyces scabies that is inducible by zinc. J Bacteriol. 1987 May;169(5):1967–1971. doi: 10.1128/jb.169.5.1967-1971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan M. B., Crawford D. L., Pometto A. L., 3rd Isolation of lignocellulose-decomposing actinomycetes and degradation of specifically 14C-labeled lignocelluloses by six selected Streptomyces strains. Can J Microbiol. 1979 Nov;25(11):1270–1276. doi: 10.1139/m79-200. [DOI] [PubMed] [Google Scholar]
- Pridham T. G., Gottlieb D. The Utilization of Carbon Compounds by Some Actinomycetales as an Aid for Species Determination. J Bacteriol. 1948 Jul;56(1):107–114. doi: 10.1128/jb.56.1.107-114.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Roegner V., Becker F. F. The quantitation of rat serum esterases by densitometry of acrylamide gels stained for enzyme activity. Anal Biochem. 1975 May 26;66(1):206–212. doi: 10.1016/0003-2697(75)90738-1. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]