Abstract
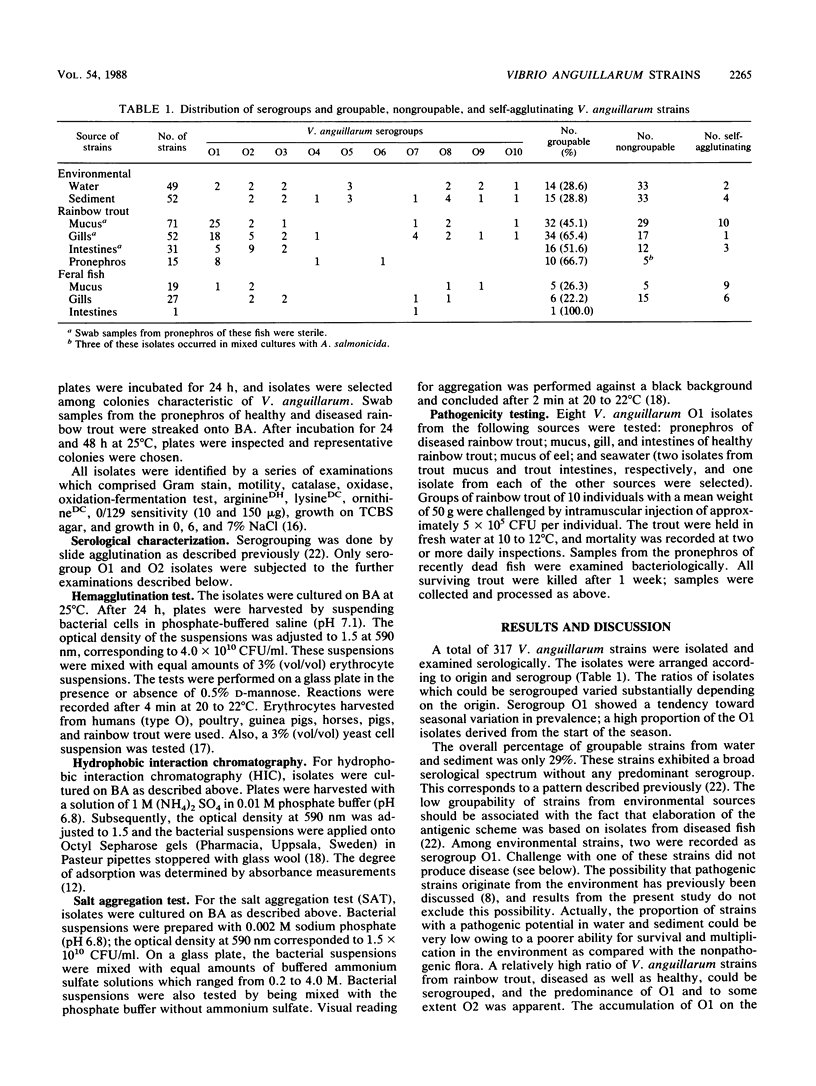
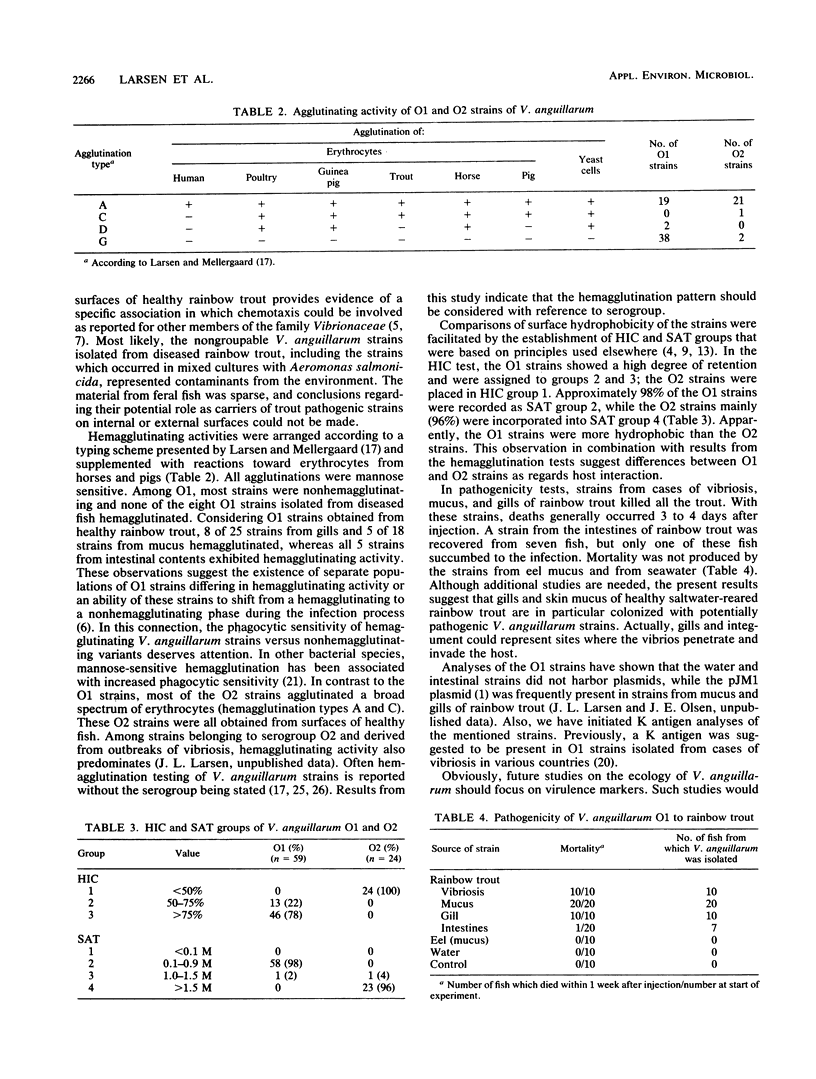
A total of 317 Vibrio anguillarum strains were isolated from water, sediment, and diseased as well as healthy rainbow trout at a Danish mariculture farm and from feral fish caught close to the farm. All strains were examined serologically. Ten sera permitted determination of the O group in 66.7% of the strains from diseased rainbow trout. Furthermore, the O group could be determined in 45.1 to 65.4% of the strains from mucus, gills, and intestinal contents of healthy rainbow trout, while only 22.2 to 28.8% of the isolates from water, sediment, and gills or mucus of feral fish were groupable. Serogroup O1 and to some extent O2 appeared to be associated with trout. Strains from these serogroups were selected for analyses of hemagglutinating activity and surface hydrophobicity. Serogroup O1 comprised hemagglutinating as well as nonhemagglutinating strains; from cases of vibriosis, all O1 strains were nonhemagglutinating. The strains belonging to serogroup O2 were generally hemagglutinating. Examinations of surface hydrophobicity by salt aggregation and hydrophobic interaction chromatography suggested that the O1 strains were more hydrophobic than the O2 strains. In pathogenicity tests, O1 strains isolated from gills and mucus of healthy rainbow trout killed all trout in the test groups. A strain from the intestinal contents of healthy rainbow trout did not produce significant mortality. This strain could, however, be frequently reisolated from the pronephros of fish in the test group concerned. After challenge with strains from eel mucus and seawater, mortality was not produced, and furthermore, these strains could not be reisolated from the pronephros.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crosa J. H., Hodges L. L., Schiewe M. H. Curing of a plasmid is correlated with an attenuation of virulence in the marine fish pathogen Vibrio anguillarum. Infect Immun. 1980 Mar;27(3):897–902. doi: 10.1128/iai.27.3.897-902.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Halstead S. A. Adhesion and chemotaxis as determinants of bacterial association with mucosal surfaces. Adv Exp Med Biol. 1978;107:429–437. doi: 10.1007/978-1-4684-3369-2_48. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Jann K., Schmidt G., Blumenstock E., Vosbeck K. Escherichia coli adhesion to Saccharomyces cerevisiae and mammalian cells: role of piliation and surface hydrophobicity. Infect Immun. 1981 May;32(2):484–489. doi: 10.1128/iai.32.2.484-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Larsen J. L., Mellergaard S. Agglutination Typing of Vibrio anguillarum Isolates from Diseased Fish and from the Environment. Appl Environ Microbiol. 1984 Jun;47(6):1261–1265. doi: 10.1128/aem.47.6.1261-1265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J. L. Vibrio anguillarum: a comparative study of fish pathogenic, environmental, and reference strains. Acta Vet Scand. 1983;24(4):456–476. doi: 10.1186/BF03546718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Pacha R. E., Kiehn E. D. Characterization and relatedness of marine vibrios pathogenic to fish: physiology, serology, and epidemiology. J Bacteriol. 1969 Dec;100(3):1242–1247. doi: 10.1128/jb.100.3.1242-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Interaction of bacterial pili and leukocytes. Infection. 1983 Jul-Aug;11(4):235–238. doi: 10.1007/BF01641208. [DOI] [PubMed] [Google Scholar]
- Sørensen U. B., Larsen J. L. Serotyping of Vibrio anguillarum. Appl Environ Microbiol. 1986 Mar;51(3):593–597. doi: 10.1128/aem.51.3.593-597.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Courtice I. D., Khouri A. G., Crosa J. H., Schiewe M. H. Serum resistance and hemagglutination ability of marine vibrios pathogenic for fish. Infect Immun. 1981 Dec;34(3):702–707. doi: 10.1128/iai.34.3.702-707.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]


