Abstract
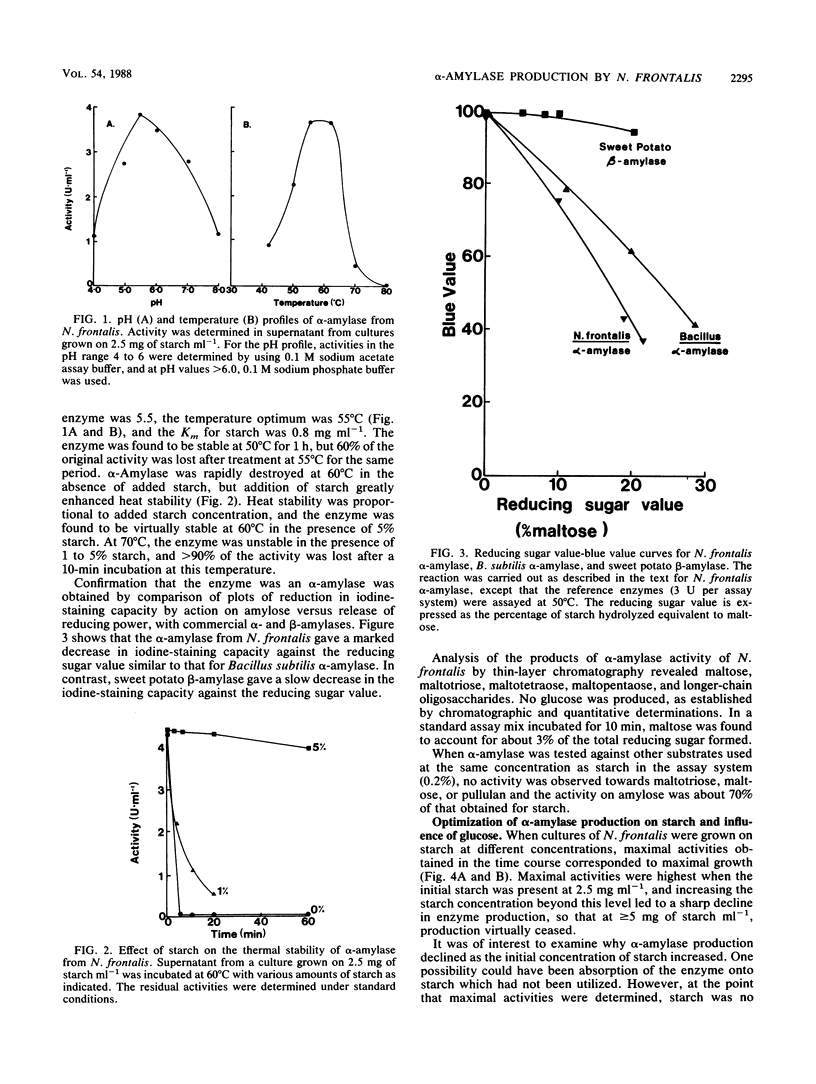
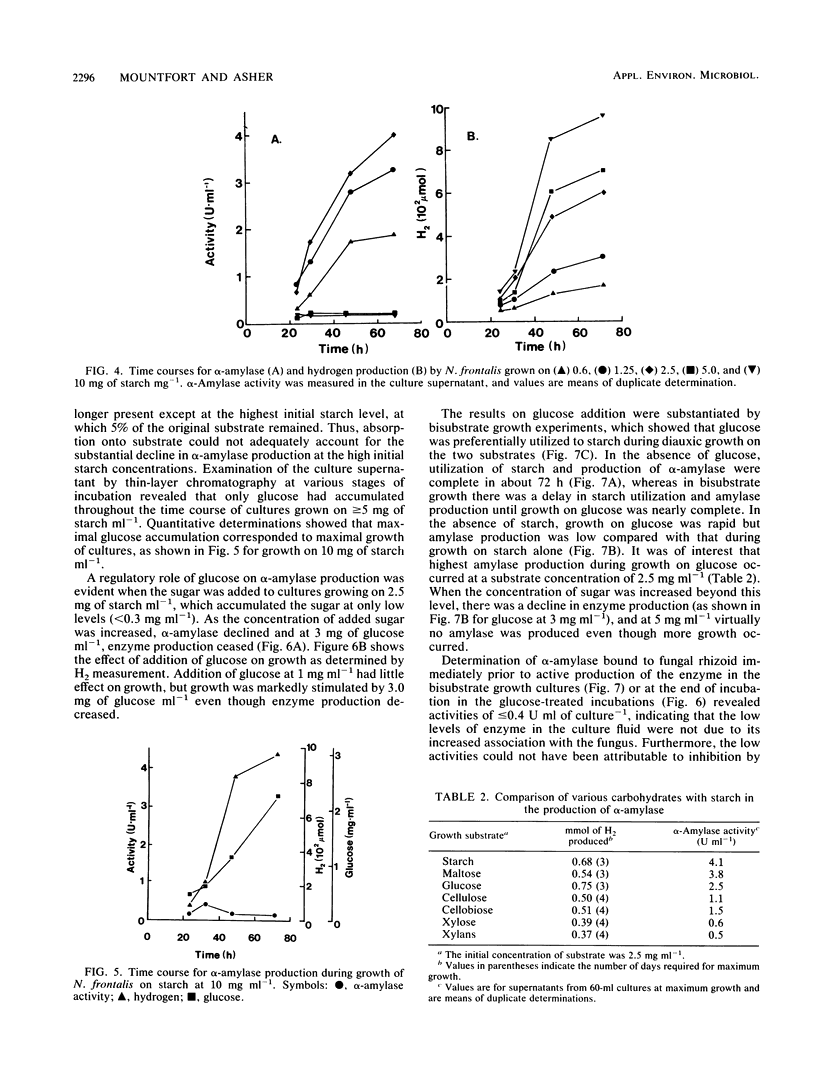
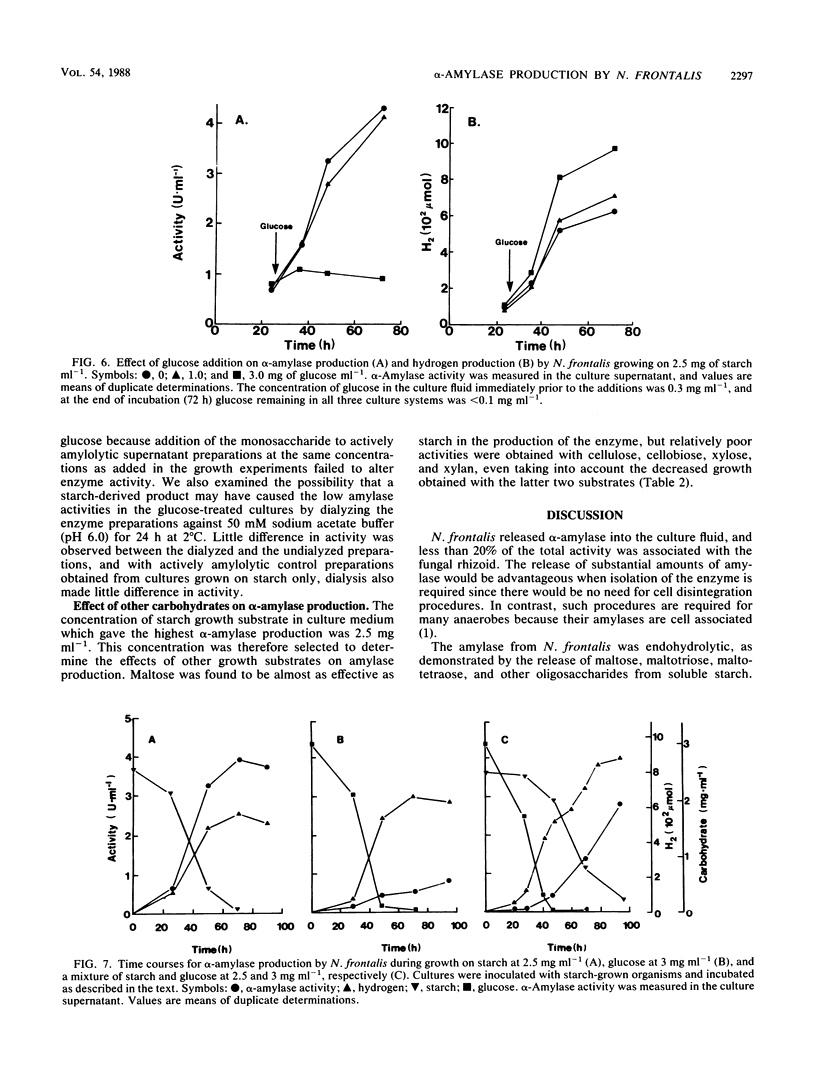
α-Amylase production was examined in the ruminal anaerobic fungus Neocallimastix frontalis. The enzyme was released mainly into the culture fluid and had temperature and pH optima of 55°C and 5.5, respectively, and the apparent Km for starch was 0.8 mg ml−1. The products of α-amylase action were mainly maltotriose, maltotetraose, and longer-chain oligosaccharides. No activity of the enzyme was observed towards these compounds or pullulan, but activity on amylose was similar to starch. Evidence for the endo action of α-amylase was also obtained from experiments which showed that the reduction in iodine-staining capacity and release in reducing power by action on amylose was similar to that for commercial α-amylase. Activities of α-amylase up to 4.4 U ml−1 (1 U represents 1 μmol of glucose equivalents released per min) were obtained for cultures grown on 2.5 mg of starch ml−1 in shaken cultures. No growth occurred in unshaken cultures. With elevated concentrations of starch (>2.5 mg ml−1), α-amylase production declined and glucose accumulated in the cultures. Addition of glucose to cultures grown on low levels of starch, in which little glucose accumulated, suppressed α-amylase production, and in bisubstrate growth studies, active production of the enzyme only occurred during growth on starch after glucose had been preferentially utilized. When cellulose, cellobiose, glucose, xylan, and xylose were tested as growth substrates for the production of α-amylase (initial concentration, 2.5 mg ml−1), they were found to be less effective than starch, but maltose was almost as effective. The fungal α-amylase was found to be stable at 60°C in the presence of low concentrations of starch (≤5%), suggesting that it may be suitable for industrial application.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antranikian G., Herzberg C., Gottschalk G. Production of Thermostable alpha-Amylase, Pullulanase, and alpha-Glucosidase in Continuous Culture by a New Clostridium Isolate. Appl Environ Microbiol. 1987 Jul;53(7):1668–1673. doi: 10.1128/aem.53.7.1668-1673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore V., Caporale C., De Rosa M., Gambacorta A. Stable, inducible thermoacidophilic alpha-amylase from Bacillus acidocaldarius. J Bacteriol. 1976 Nov;128(2):515–521. doi: 10.1128/jb.128.2.515-521.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A. Amylolytic activity of selected species of ruminal bacteria. Appl Environ Microbiol. 1988 Mar;54(3):772–776. doi: 10.1128/aem.54.3.772-776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mot R., Verachtert H. Purification and Characterization of Extracellular Amylolytic Enzymes from the Yeast Filobasidium capsuligenum. Appl Environ Microbiol. 1985 Dec;50(6):1474–1482. doi: 10.1128/aem.50.6.1474-1482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON P. N., MACPHERSON M. Amylases of Clostridium butyricum and a Streptococcus isolated from the rumen of the sheep. Biochem J. 1952 Dec;52(4):671–679. doi: 10.1042/bj0520671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Extracellular beta-Amylase from Clostridium thermosulfurogenes. Appl Environ Microbiol. 1985 May;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Pullulanase and Glucoamylase from Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1168–1173. doi: 10.1128/aem.49.5.1168-1173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Simultaneous and Enhanced Production of Thermostable Amylases and Ethanol from Starch by Cocultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1174–1181. doi: 10.1128/aem.49.5.1174-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan T., Chandra A. K. Purification and Characterization of alpha-Amylase from Bacillus licheniformis CUMC305. Appl Environ Microbiol. 1983 Aug;46(2):430–437. doi: 10.1128/aem.46.2.430-437.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda S., Chandra A. K. New strains of Bacillus licheniformis and Bacillus coagulans producing thermostable alpha-amylase active at alkaline pH. J Appl Bacteriol. 1980 Feb;48(1):47–58. doi: 10.1111/j.1365-2672.1980.tb05205.x. [DOI] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1985 May;49(5):1314–1322. doi: 10.1128/aem.49.5.1314-1322.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N. A thermophilic extracellular -amylase from Bacillus licheniformis. Arch Biochem Biophys. 1973 Apr;155(2):290–298. doi: 10.1016/0003-9861(73)90117-3. [DOI] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- WALKER G. J. THE CELL-BOUND ALPHA-AMYLASES OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:289–298. doi: 10.1042/bj0940289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Hope P. M. Degradation of starch granules by some amylolytic bacteria from the rumen of sheep. Biochem J. 1964 Feb;90(2):398–408. doi: 10.1042/bj0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]



