Abstract
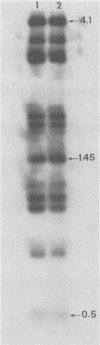
Electroporation-induced transformation of intact cells of Clostridium perfringens 3624A with plasmids pAMB1 and pHR106 resulted in 3.8 X 10(-5) and 4.2 X 10(-4) transformants per viable cell, respectively. With respect to shuttle plasmid pHR106, these values represent a greater than 100-fold increase in transformation frequency when compared with the values reported with polyethylene glycol-induced L-phase variants.
Full text
PDF
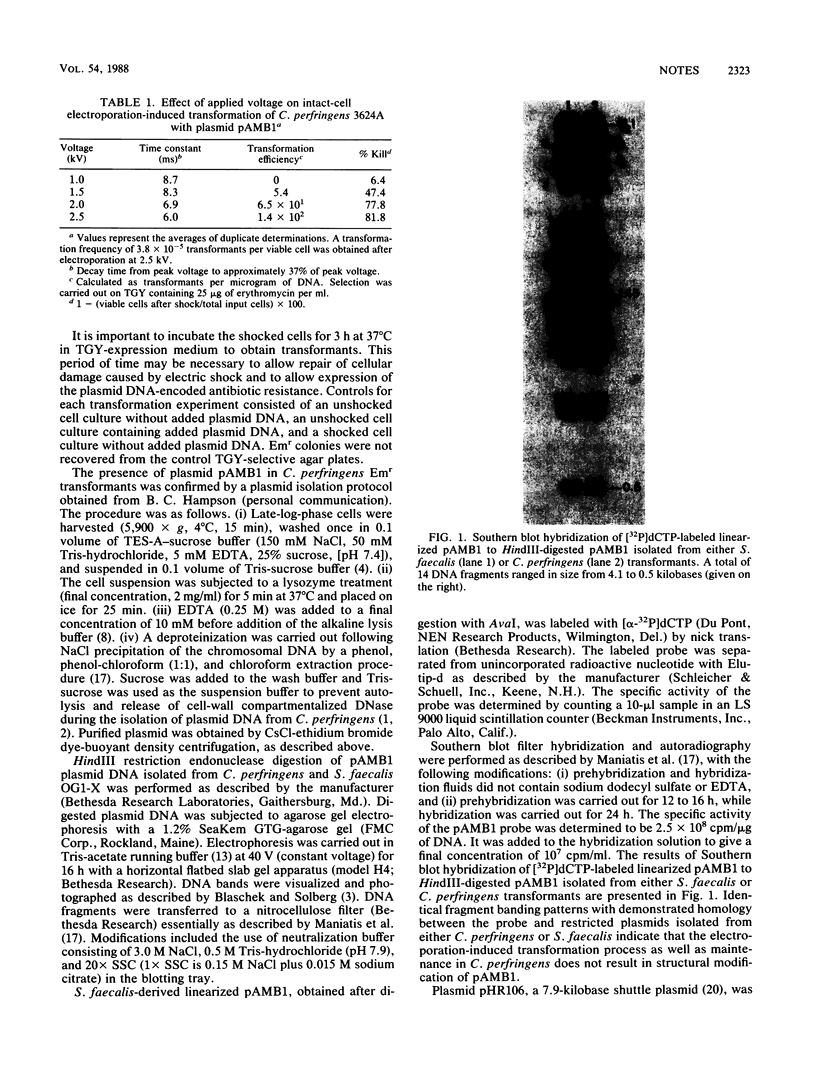

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaschek H. P., Klacik M. A. Development of a cell wash buffer that minimizes nucleic acid loss from Clostridium perfringens 10543 A. Can J Microbiol. 1985 Jun;31(6):575–578. doi: 10.1139/m85-107. [DOI] [PubMed] [Google Scholar]
- Blaschek H. P., Klacik M. A. Role of DNase in recovery of plasmid DNA from Clostridium perfringens. Appl Environ Microbiol. 1984 Jul;48(1):178–181. doi: 10.1128/aem.48.1.178-181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschek H. P., Solberg M. Isolation of a plasmid responsible for caseinase activity in Clostridium perfringens ATCC 3626B. J Bacteriol. 1981 Jul;147(1):262–266. doi: 10.1128/jb.147.1.262-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefort G., Magot M., Ionesco H., Sebald M. Characterization and transferability of Clostridium perfringens plasmids. Plasmid. 1977 Nov;1(1):52–66. doi: 10.1016/0147-619x(77)90008-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heefner D. L., Squires C. H., Evans R. J., Kopp B. J., Yarus M. J. Transformation of Clostridium perfringens. J Bacteriol. 1984 Aug;159(2):460–464. doi: 10.1128/jb.159.2.460-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionesco H., Bieth G., Dauguet C., Bouanchaud D. Isolement et identification de deux plasmides d'une souche bactériocinogène de Clostridium perfringens. Ann Microbiol (Paris) 1976 Oct;127B(3):283–294. [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Blaschek H. P. Transformation of Heat-Treated Clostridium acetobutylicum Protoplasts with pUB110 Plasmid DNA. Appl Environ Microbiol. 1984 Oct;48(4):737–742. doi: 10.1128/aem.48.4.737-742.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Simple method for demonstrating small plasmid deoxyribonucleic acid molecules in oral streptococci. Appl Environ Microbiol. 1980 May;39(5):1070–1073. doi: 10.1128/aem.39.5.1070-1073.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony D. E., Mader J. A., Dubel J. R. Transformation of Clostridium perfringens L forms with shuttle plasmid DNA. Appl Environ Microbiol. 1988 Jan;54(1):264–267. doi: 10.1128/aem.54.1.264-267.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid S. J., Allcock E. R., Jones D. T., Woods D. R. Transformation of Clostridium acetobutylicum Protoplasts with Bacteriophage DNA. Appl Environ Microbiol. 1983 Jan;45(1):305–307. doi: 10.1128/aem.45.1.305-307.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I., Holmes W. M., Hylemon P. B. Development of a new shuttle plasmid system for Escherichia coli and Clostridium perfringens. Appl Environ Microbiol. 1988 Jan;54(1):268–270. doi: 10.1128/aem.54.1.268-270.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Clewell D. B. Return of Streptococcus faecalis DNA cloned in Escherichia coli to its original host via transformation of Streptococcus sanguis followed by conjugative mobilization. J Bacteriol. 1984 Dec;160(3):1109–1114. doi: 10.1128/jb.160.3.1109-1114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., Heefner D. L., Evans R. J., Kopp B. J., Yarus M. J. Shuttle plasmids for Escherichia coli and Clostridium perfringens. J Bacteriol. 1984 Aug;159(2):465–471. doi: 10.1128/jb.159.2.465-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar I. P., Neumann E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys Chem. 1984 May;19(3):211–225. doi: 10.1016/0301-4622(84)87003-9. [DOI] [PubMed] [Google Scholar]