Abstract
This paper describes the identification of a new family of mammalian genes that encode secreted proteins containing homology to the cysteine-rich ligand-binding domain found in the frizzled family of transmembrane receptors. The secreted frizzled-related proteins (sFRPs) are approximately 30 kDa in size, and each contains a putative signal sequence, a frizzled-like cysteine-rich domain, and a conserved hydrophilic carboxy-terminal domain. The sFRPs are not the products of differential splicing of the known frizzled genes. Glycosylphosphatidylinositol-anchored derivatives of sFRP-2 and sFRP-3 produced in transfected human embryonic kidney cells confer cell-surface binding by the Drosophila Wingless protein. These observations suggest that sFRPs may function in vivo to modulate Wnt signaling, or, alternatively, as novel ligands for as yet unidentified receptors.
The frizzled gene is one of a group of genes in Drosophila that control tissue polarity (1, 2). Mutations in tissue polarity genes cause a disorganization in the polarity of cuticular structures, such as bristles and hairs, with respect to the body axes of the animal. The product of the frizzled gene is an integral membrane protein with an extracellular cysteine-rich domain (CRD) followed by seven putative membrane-spanning segments (3). This structure suggests that frizzled is a receptor for one or more ligands that carry tissue polarity information. Recently, a large number of frizzled-related genes have been identified in diverse animals, including mammals, birds, fish, sea urchins, and nematodes (4–6). A second frizzled-related gene was also identified in Drosophila, and is referred to as frizzled-2 (Dfz2; ref. 7). Each of these homologues encodes a protein with all of the characteristic features of the original Drosophila frizzled protein.
Experiments in which Dfz2 coding sequences were introduced into Drosophila tissue culture cells reveal that it can function as a receptor for Wingless, one member of the Wnt family of extracellular signaling molecules (7). Drosophila Schneider cells transfected with Dfz2 become responsive to Wingless, and they bind Wingless protein at the cell surface. Transfection of Dfz2 or a subset of mammalian frizzled family members also confers cell surface Wingless binding to a human embryonic kidney cell line (293 cells). Further evidence that Wnt and frizzled proteins are linked in receptor-ligand relationships has come from injection experiments in Xenopus embryos, in which expression of various mammalian frizzled proteins was found to modulate the developmental effects of a subset of Wnt molecules and to alter the subcellular distribution of Wnt proteins (8, 9).
As noted above, each frizzled protein contains at its amino terminus a conserved CRD. The CRD is joined to the transmembrane domain by a divergent protein segment that is predicted to have an extended and flexible structure. The CRD is required for Wingless binding to the surface of transfected cells, as shown by the ability of Wingless to bind to cells expressing a glycosylphosphatidylinositol (GPI)-anchored Dfz2 CRD in the absence of its transmembrane domain, and by the inability of Wingless to bind to cells expressing a mammalian frizzled protein with the CRD removed (7). The CRD motif has also been found in one of the nonhelical domains of an atypical collagen (10), and, in variant forms, in the amino-terminal region of the Drosophila and mammalian Smoothened proteins, frizzled-like membrane proteins that, together with the Patched protein, appear to mediate Hedgehog signaling (11–14).
In this paper we describe the identification in mammals of a family of proteins that consist of a signal sequence followed by a frizzled-like CRD and a small hydrophilic carboxy-terminal domain. Membrane-anchored derivatives of two of these proteins confer cell surface binding to Drosophila Wingless, suggesting that these proteins may act in vivo to modulate Wnt signaling.
MATERIALS AND METHODS
cDNA and Genomic DNA Clones.
cDNA clones encoding secreted frizzled-related protein (sFRP)-1, sFRP-2, and sFRP-3 were isolated from an oligo(dT)-primed P0-P7 mouse eye cDNA library (Lanahan, A., Sun, H., and J.N. unpublished data) by DNA hybridization with human expressed sequence tag (EST) probes under standard conditions (15). The complete coding regions of sFRP-1, sFRP-2, and sFRP-3 were sequenced from each of two independent full-length cDNA clones. The mouse sFRP-4 genomic clone was obtained from a partial MboI digest mouse genomic DNA library in bacteriophage λ by cross-hybridization with a sFRP-3 cDNA probe.
Northern Blot Hybridization and RNase Protection.
Total RNA was prepared from the indicated adult mouse and rat tissues by homogenization in guanidinium thiocyanate (16). Twenty micrograms of total rat RNA was resolved by agarose gel electrophoresis in the presence of formaldehyde and hybridized with sFRP-1, sFRP-2, or sFRP-3 coding region probes in 50% formamide at 37°C. Filters were washed in 0.2× standard saline citrate (SSC) at 68°C. Ten micrograms of total RNA from each tissue or 10 μg of yeast tRNA was used for the RNase protection assay. Riboprobes were synthesized using T3 RNA polymerase on linearized templates in pBluescript (Stratagene). Each mouse sFRP probe contained 150–250 bases from the antisense strand linked to 25–50 bases of vector sequence. Reagents for RNase protection were obtained from Ambion (Austin, TX), and the hybridization and digestion conditions were as recommended by Ambion.
In Situ Hybridization.
Freshly dissected adult mouse brains and eyes, whole embryos, or heads were rapidly frozen in plastic molds placed on a dry ice/ethanol slurry and processed for sectioning as previously described (17). 33P-labeled antisense riboprobes were prepared from linearized pBluescript plasmid subclones using T3 RNA polymerase. In situ hybridization was performed in 50% formamide/0.3 M NaCl at 56°C as described (18). Following RNase treatment the slides were washed for 1 hr in 0.1× SSC at 55°C. The hybridized sections were exposed to x-ray film, and the slides were stained with cresyl violet. Digitized images of the stained slides and corresponding autoradiograms were superimposed using adobe photoshop software. The sFRP-1 probe contained a 585-bp insert from codon 49 to codon 244.
Interspecific Mouse Backcross Mapping.
Interspecific backcross progeny were generated by mating (C57BL/6J × Mus spretus) F1 females and C57BL/6J males as described (19). A total of 205 N2 mice were used to map the sFRP loci. DNA isolation, restriction enzyme digestion, agarose gel electrophoresis, Southern blot transfer, and hybridization were performed essentially as described (20). All blots were prepared with Zetabind nylon membrane (AMF Cuno). Probes specific for each locus were labeled with [α-32P]dCTP using a random priming labeling kit (Stratagene); washing was done to a final stringency of 0.5–1.0× SSCP (1× SSCP = 120 mM NaCl/5 mM sodium citrate/20 mM sodium phosphate, pH 6.8), 0.1% SDS, 65°C. The sFRP-l probe detected SacI fragments of 7.4, 4.8, and 3.4 kb in C57BL/6J (B) DNA and 5.8 and 3.8 kb in M. spretus (S) DNA. The sFRP-2 probe detected HindIII fragments of 7.1 and 0.6 kb (B) and 9.1 and 0.6 kb (S). The sFRP-3 probe detected major SacI fragments of 5.1 and 0.7 (B) and 2.1 and 1.6 (S) kb. The sFRP-4 probe detected major SacI fragments of 2.7 (B) and 3.3 (S) kb. The presence or absence of M. spretus-specific fragments was followed in backcross mice. When more than one fragment was polymorphic, the fragments cosegregated.
A description of most of the probes and restriction fragment length polymorphisms (RFLPs) for the loci linked to the Sfrp loci in the interspecific backcross has been reported. These include: Hoxd, Sfpil, and Rag1 on chromosome 2 (21, 22); Fgg, Ntrkl, and Lor on chromosome 3 (23, 24); Col4al, Plat, and Fgfrl on chromosome 8 (25, 26); and Nid, Amph, and Btn on chromosome 13 (27). The probe for Mme, an ≈200-bp fragment of mouse cDNA that was kindly provided by Eli Keshet (Hebrew University, Jerusalem), has not been reported previously for this interspecific backcross. This probe detected major PstI fragments of 5.0 kb (B) and 4.2 kb (S). The inheritance of the 4.2-kb M. spretus-specific PstI RFLP was followed. Recombination distances were calculated as described (28) using the computer program spretus madness. Gene order was determined by minimizing the number of recombination events required to explain the allele distribution patterns.
Production of Recombinant sFRP.
sFRP proteins and their derivatives were produced by transient transfection of 293 cells using the pCIS vector (29). Constructs for expression of GPI-anchored proteins were prepared by engineering a unique HindIII site (sFRP-1 and sFRP-2) or a unique EcoRV site (sFRP-3) immediately upstream of the stop codon. These sites were joined to synthetic DNA encoding a myc epitope (30) flanked by glycine spacers (GGGMEQKLISEEDLNGGG), followed by a DNA segment encoding the carboxy-terminal 40 amino acids of decay-activating factor (31). An analogous set of constructs was also prepared with a carboxy-terminal myc epitope followed by a stop codon. Constructs for expression of alkaline phosphatase fusion proteins were prepared by joining the sFRP coding regions upstream of the human placental alkaline phosphatase coding region in a modified version of pAPtag2, a vector from which the alkaline phosphatase signal sequence has been removed (32). Cell surface expression of GPI-anchored proteins was monitored by staining intact cells with anti-myc mAb 9E10 (30) followed by a fluorescent secondary antibody as described in ref. 7. The yield of secreted sFRP-alkaline phosphatase fusion proteins was determined by collecting conditioned medium from transfected 293 cells and measuring alkaline phosphatase enzyme activity with the colorimetric substrate p-nitrophenylphosphate (33). The yield of secreted sFRP with a carboxy-terminal myc epitope tag was determined by Western blotting of conditioned medium with mAb 9E10.
Wingless Binding.
Cell-surface binding of Wingless protein to transfected 293 cells was carried out as described in ref. 7. In brief, transfected cells were grown for 16 hr in 10 mM chlorate and treated with 20 mU heparatinase for 3 hr to decrease the extracellular matrix, incubated with conditioned medium containing Wingless produced from transfected Schneider (S2) cells, washed, and fixed with paraformaldehyde. Bound Wingless protein was visualized by confocal microscopy following incubation with affinity-purified rabbit anti-Wingless antibodies and fluorescent secondary antibody.
RESULTS
Identification of sFRPs.
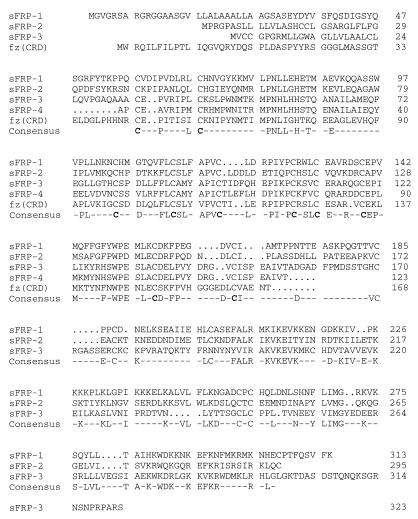
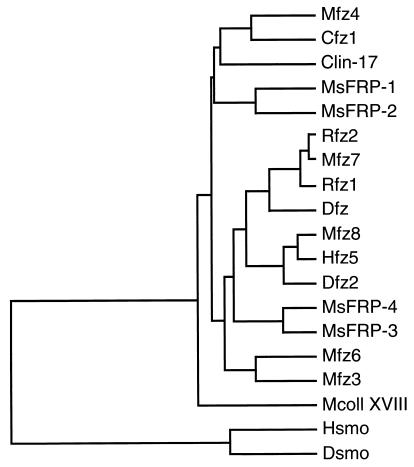
A search (34) of the EST database using a number of frizzled sequences as queries revealed three human ESTs (ref. 35; ID numbers 159365, 182765, and 51061) with significant homology to the CRD found near the amino terminus of each frizzled sequence. To define the primary structures of these frizzled-related sequences, cDNA clones containing mouse orthologues were isolated by screening a developing mouse eye cDNA library with the human EST probes. One exon encoding part of a putative fourth homologue was identified as a cross-hybridizing segment in a mouse genomic library. Each of these four sequences revealed a putative amino-terminal signal sequence followed by a region of approximately 110 amino acids with a high degree of similarity to the frizzled CRD consensus, including complete conservation of the 10 cysteines. Carboxy-terminal to the CRD, the three full-length sequences share a related hydrophilic region of approximately 175 amino acids. No homologies were observed between these new protein sequences and the frizzled proteins outside of the CRD. These four predicted proteins will be referred to hereafter as sFRPs. The dendrogram in Fig. 1 shows that the CRD sequences of the sFRPs form two branches within the tree of CRDs thus far identified. The many differences between the sFRP and frizzled CRDs indicate that the sFRPs have not arisen by differential splicing of the known frizzled genes. While the present work was in progress, a cDNA clone encoding mouse sFRP-2, referred to as SDF5, was identified from a bone marrow stromal cell line using a signal sequence trap (36), and cDNA clones encoding bovine and human sFRP-3, referred to as FRZB, were identified from cartilage (37). The FRZB cDNAs predict proteins with 92% identity to the murine sFRP-3 amino acid sequence reported here.
Figure 1.
(Left) Alignment of mouse sFRP amino acid sequences. The alignment includes the CRD of Drosophila frizzled (3), indicated by fz(CRD). The sFRP-4 sequence is incomplete; it is derived from a segment of genomic DNA that encompasses a single exon of the sFRP-4 gene. (Right) Dendrogram of CRD sequences in which the length of each horizontal line is proportional to the degree of amino acid sequence divergence. The region used to construct the dendrogram extends from the 1st to the 10th cysteine of the CRD. smo, smoothened; coll XVIII, collagen XVIII. Species origins are indicated by an uppercase letter: C, C. elegans; D, Drosophila; H, human; M, mouse; R, rat.
Tissue Distribution of sFRP Transcripts.
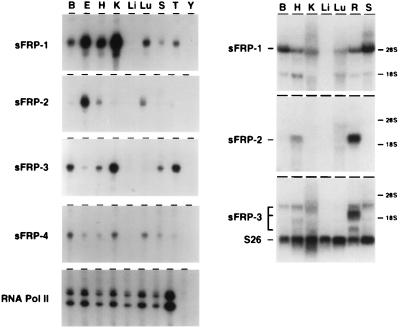
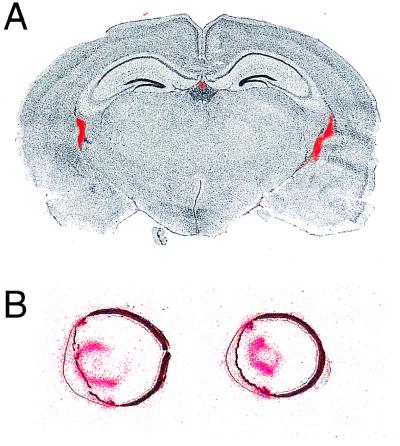
The distributions of sFRP transcripts were determined by RNase protection, Northern blot hybridization, and in situ hybridization (Figs. 2 and 3). In adult mice and rats, sFRP-1 and sFRP-3 transcripts are readily detected in multiple tissues. The sFRP-3 probe reveals multiple transcripts in the retina. The expression pattern of sFRP-2 is noteworthy for the high levels found in the retina relative to other tissues examined (Fig. 2). sFRP-4 transcripts are found only at low levels in multiple tissues. Apparent differences in tissue distribution between the mouse and rat samples probably represent bona fide species differences, although we cannot rule out the possibility that some of the hybridizing bands seen on Northern blots represent cross-hybridization with as yet uncharacterized sFRP family members. For sFRP-1, in situ hybridization revealed a distribution of transcripts in the adult mouse brain and eye that is more restricted than can be inferred from the RNase protection or Northern blot hybridization analyses (Fig. 3). In the brain, sFRP-1 is expressed exclusively in the choroid plexus, and in the eye it is expressed principally in the ciliary body and the anterior epithelium of the lens. Each of these structures transports ions and metabolites to maintain the composition of the cerebrospinal fluid, the aqueous humor, and the lens, respectively. Interestingly, Mfz4 transcripts are also localized to the choroid plexus (5).
Figure 2.
Tissue distribution of sFRP transcripts in the adult mouse and rat. (Left) Ten micrograms of total mouse RNA from the indicated tissues was used for RNase protection with each of the sFRP probes. A control reaction with a RNA polymerase II probe is shown at the bottom. The sFRP-4 RNase protection autoradiogram was exposed three times longer than the other autoradiograms. (Right) Twenty micrograms of total rat RNA from the indicated tissues was used for Northern blot hybridization with each of the sFRP probes. A control probe derived from ribosomal protein S26 is shown at the bottom of the sFRP-3 Northern blot. The sFRP-4 probe failed to produce a detectable signal under the hybridization conditions used. The mobilities of 18S and 28S ribosomal RNAs are indicated to the right. B, brain; E, eye; H, heart; K, kidney; Li, liver; Lu, lung; R, retina; S, spleen; T, testis; Y, yeast tRNA.
Figure 3.
In situ localization of sFRP-1 transcripts in the adult mouse brain and eye. 33P in situ hybridization is shown in red, superimposed upon a cresyl violet stain shown in black and white. (A) In the brain, the hybridization signal is seen in the two lateral ventricles and in the central third ventricle. (B) In the eye, the hybridization signal is seen in the anterior epithelium of the lens and in the ciliary body. The cornea is facing to the left in both sections.
Chromosomal Localization of sFRP Genes.
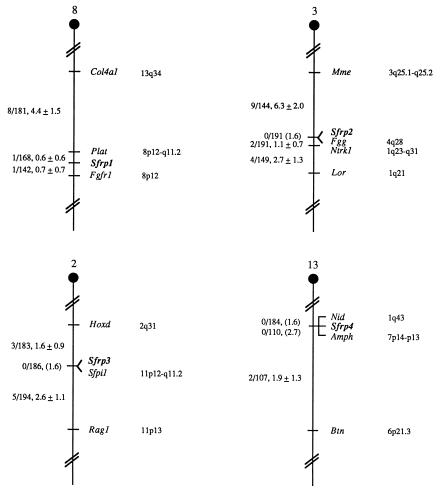
The chromosomal locations of the four sFRP genes were determined in the mouse using an interspecific backcross mapping panel derived from crosses of [(C57BL/6J × M. spretus)F1 × C57BL/6J] mice. This mapping panel has been typed for over 2,300 loci that are well dispersed among all the autosomes and the X chromosome (19). cDNA fragments specific for each of the sFRP loci were used as probes in Southern blot hybridization analysis of C57BL/6J and M. spretus genomic DNAs that were separately digested with several different restriction enzymes to identify informative RFLPs useful for gene mapping (see Materials and Methods). The strain distribution pattern of each RFLP in the interspecific backcross was then determined by following the presence or absence of RFLPs specific for M. spretus in backcross mice. Each of these genes mapped to a single chromosomal location. The mapping results assigned the four loci to four different mouse autosomes, indicating that the sFRP genes have been dispersed during evolution (Fig. 4).
Figure 4.
Partial chromosome linkage maps showing the locations of the sFRP-1, sFRP-2, sFRP-3, and sFRP-4 genes in the mouse. The genes were mapped by interspecific backcross analysis. To the left of each chromosome map, the number of recombinant N2 animals divided by the total number of N2 animals typed for each pair of loci is presented. The recombination frequencies, expressed as genetic distance in centimorgans (± one standard error) are also shown. The upper 95% confidence limit of the recombination distance is given in parentheses when no recombinants were found between loci. Gene order was determined by minimizing the number of recombination events required to explain the allele distribution patterns. The positions of loci on human chromosomes, where known, are shown to the right of the chromosome maps. References for the map positions of most human loci can be obtained from the Genome Data Base, a computerized database of human linkage information maintained by The William H. Welch Medical Library of The Johns Hopkins University (Baltimore, MD).
The sFRP-1 gene (Sfrp1) mapped to the proximal region of mouse chromosome 8, 0.6 cM distal to Plat and 0.7 cM proximal to Fgfr1. The sFRP-2 gene (Sfrp2) mapped to the central region of mouse chromosome 3, and did not recombine with Fgg in 191 mice typed in common. This suggests that the two loci are within 1.6 cM of each other (upper 95% confidence interval). The sFRP-3 gene (Sfrp3) mapped to the central region of mouse chromosome 2 and did not recombine with Sfpi1 in 186 mice typed in common, suggesting the two loci are within 1.6 cM of each other. Finally, the sFRP-4 gene (Sfrp4) mapped to the proximal region of mouse chromosome 13 and did not recombine with Nid (0/184) or Amph (0/110), suggesting that Sfrp4 is within 1.6 cM of Nid and 2.7 cM of Amph. The sFRP genes map to chromosomal locations that are different from the locations of any known frizzled genes (5).
The human chromosomal locations of the Sfrp loci can be predicted based on known mouse–human linkage homologies (Fig. 4). For example, Plat has been assigned to human chromosome 8p12-q11.2. The tight linkage between Sfrp1 and Plat in the mouse suggests that the human homologue of Sfrp1 resides in this region as well. Similarly, Sfrp2 is likely to map to human chromosome 4q, Sfrp3 to 11p, and Sfrp4 to either 1q or 7p.
sFRP-2 and sFRP-3 Confer Wingless Binding in Transfected Cells.
To examine the possibility that the CRD domain from one or more of the sFRPs interacts with members of the Wnt protein family, we determined whether Drosophila Wingless binds to cells expressing cell-surface derivatives of the sFRPs. For this experiment, the sFRP proteins were anchored to the plasma membrane of transiently transfected cells via a GPI moeity. The expression constructs consisted of a segment of cDNA encoding the carboxy terminus of decay activating factor (DAF), a GPI-anchored protein (31), joined to the penultimate codon of the sFRP open reading frame. A DNA segment encoding a myc epitope was inserted between the sFRP coding region and the DAF segment so that the level and subcellular localization of each sFRP fusion protein could be monitored. Immunostaining of intact 293 cells transfected with sFRP-2/myc/DAF or sFRP-3/myc/DAF with anti-myc mAb 9E10 (30) revealed a high level of immunoreactivity at the plasma membrane, while untransfected 293 cells or 293 cells transfected with sFRP-1/myc/DAF showed no detectable plasma membrane immunoreactivity. This observation suggests that in this expression system, sFRP-1/myc/GPI is not efficiently folded and/or transported through the endoplasmic reticulum–Golgi secretory pathway.
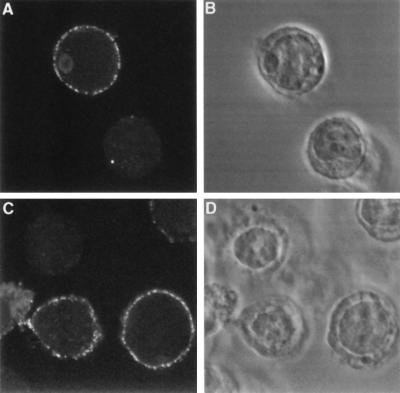
293 cells transiently transfected with each of the sFRP/myc/DAF fusions were incubated with soluble Wingless protein, washed, fixed, and immunostained with anti-Wingless antibodies (Fig. 5). Binding of Wingless was observed with sFRP-2/myc/DAF and sFRP-3/myc/DAF transfected cells, but not with sFRP-1/myc/DAF transfected cells or untransfected cells, as indicated by the binding of anti-Wingless antibody. The observed cell-surface binding of Wingless closely resembles that seen upon transfection of Dfz2 or the subset of mammalian frizzled sequences that confer Wingless binding in this assay (7).
Figure 5.
sFRP-2 and sFRP-3 bind to Drosophila Wingless when they are expressed as GPI-anchored cell-surface proteins. 293 cells were transiently transfected either with sFRP-2 (A and B) or with sFRP-3 (C and D) expression constructs in which the sFRP open reading frames were linked at their carboxy termini to a myc epitope-tagged GPI anchor sequence. The cells were incubated with conditioned medium containing Wingless protein, immunostained with affinity purified anti-Wingless antibodies, and examined by confocal microscopy. (A and C) Immunofluorescent staining. (B and D) Phase contrast.
DISCUSSION
In this paper we describe a new gene family, referred to as the sFRP family, the members of which encode proteins containing a frizzled-like CRD. The sFRP proteins contain a consensus signal sequence at their amino termini, and they lack any obvious transmembrane segments, suggesting that they are secreted. This conjecture is supported by the observed secretion from transfected 293 cells of sFRP-2 and sFRP-3 fused at their carboxy termini to either a myc epitope or human placental alkaline phosphatase. It is also supported by the observed plasma membrane localization, presumably via a GPI anchor, of sFRP-2 and sFRP-3 fused to a segment of DAF, and by the isolation of sFRP-2 in a signal sequence trap screen (36).
In adult mice and rats, the tissue distribution of sFRP expression is most notable for the high level of sFRP-2 RNA in the retina relative to other tissues, and the concentration of sFRP-1 RNA in tissues involved in solute transport in the eye and brain. The pattern of sFRP-1 expression suggests that the choroid plexus, ciliary body, and anterior epithelium of the lens may be the source of a common extracellular signal mediated by sFRP-1.
One possible role of the sFRP family is suggested by the ability of GPI-anchored sFRP-2 and sFRP-3 to confer cell-surface Wingless binding. The simplest interpretation of this experiment is that Wingless binds to the sFRP proteins directly, presumably via the CRD, as suggested for the frizzled proteins in analogous binding experiments with a GPI-anchored Dfz2 CRD domain (7). We note that the Wingless binding data are also consistent with models in which additional molecules, present either in the conditioned medium containing Wingless or on the surface of 293 cells, are required for Wingless binding to sFRP transfected cells. We also note that any interactions observed between mammalian sFRPs and Drosophila Wingless are likely to reflect only the most generic type of interaction between these protein families. It remains to be determined whether vertebrate sFRPs interact with vertebrate Wnt proteins, and, if so, whether this interaction is meaningful in vivo. If subsequent experiments demonstrate such an interaction, then it is possible that the sFRPs might alter the biosynthesis, stability, or spatial distribution of Wnt proteins, or might modulate Wnt action by competing with binding to frizzled receptors. An alternative model of sFRP action envisions an interaction of sFRPs with as yet unidentified receptors on target cells. In both models, sFRPs act as extracellular ligands to convey information between cells.
Acknowledgments
We thank Ingrid Caras for a cDNA clone-encoding decay-activating factor; Roel Nusse and Cindy Harryman-Samos for gifts of S2 HS-Wg cells and anti-Wg antibodies; John Flanagan for the alkaline phosphatase fusion vector; Se-Jin Lee for the mouse genomic DNA library; Clark Riley, Carol Davenport, Jeanine Ptak, and Monica Kazienko for oligonucleotide synthesis and automated DNA sequencing; Yanshu Wang, Don Zack, and Jinghwa Chang for helpful discussions; M. Barnstead and D. Householder for excellent technical assistance; and Purnima Bhanot, Patti Sherman, and Patrick Tong for comments on the manuscript. This research was supported by the Howard Hughes Medical Institute (A.R., J.C.H., P.M.S., and J.N.) and, in part, by the National Cancer Institute, Department of Health and Human Services, under contract with Advanced BioScience Laboratory (D.J.G., N.G.C., and N.A.J.).
ABBREVIATIONS
- CRD
cysteine-rich domain
- DAF
decay activating factor
- EST
expressed sequence tag
- GPI
glycosylphosphatidylinositol
- RFLPs
restriction fragment length polymorphisms
- sFRP
secreted frizzled-related protein
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. U88566–U88569U88566U88567U88568U88569).
References
- 1.Gubb D, Garcia-Bellido A. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 2.Adler P N, Charlton J, Vinson C. Dev Genet. 1987;8:99–119. [Google Scholar]
- 3.Vinson C, Conover S, Adler P N. Nature (London) 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- 4.Chan S D H, Karpf D B, Fowlkes M E, Hooks M, Bradley M S, Vuong V, Bambino T, Liu M Y C, Arnaud C D, Strewler G J, Nissenson R A. J Biol Chem. 1992;267:25202–25207. [PubMed] [Google Scholar]
- 5.Wang Y, Macke J P, Abella B S, Andreasson K, Worley P, Gilbert D J, Copeland N G, Jenkins N A, Nathans J. J Biol Chem. 1996;271:4468–4476. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- 6.Sawa H, Lobel L, Horvitz H R. Genes Dev. 1996;10:2189–2197. doi: 10.1101/gad.10.17.2189. [DOI] [PubMed] [Google Scholar]
- 7.Bhanot P, Brink M, Harryman Samos C, Hsieh J-C, Wang Y, Macke J P, Andrew D, Nathans J, Nusse R. Nature (London) 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 8.Yang-Snyder J, Miller J R, Brown J D, Lai C-J, Moon R T. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 9.He, X., Saint-Jeannet, J.-P., Wang, Y., Nathans, J., Dawid, I. & Varmus, H. E. (1997) Science, in press. [DOI] [PubMed]
- 10.Rehn M, Pihlajaniemi T. J Biol Chem. 1995;270:4705–4711. doi: 10.1074/jbc.270.9.4705. [DOI] [PubMed] [Google Scholar]
- 11.Alcedo J, Ayzenzon M, Van Ohlen T, Noll M, Hooper J E. Cell. 1996;86:221–232. doi: 10.1016/s0092-8674(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 12.van den Heuvel M, Ingham P W. Nature (London) 1996;382:547–551. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- 13.Stone D M, Hynes M, Armanini M, Swanson T A, Gu Q, Johnson R L, Scott M P, Pennica D, Goddard A, Phillips H, Noll M, Hooper J E, de Sauvage F, Rosenthal A. Nature (London) 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 14.Marigo V, Davey R A, Zuo Y, Cunningham J M, Tabin C J. Nature (London) 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Chomczynski P, Saachi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Cole A, Abu-Shakra S, Saffen D, Baraban J, Worley P. J Neurochem. 1990;55:1920–1927. doi: 10.1111/j.1471-4159.1990.tb05777.x. [DOI] [PubMed] [Google Scholar]
- 18.Saffen D, Cole A, Worley P, Christy B, Ryder K, Baraban J. Proc Natl Acad Sci USA. 1988;85:7795–7799. doi: 10.1073/pnas.85.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copeland N G, Jenkins N A. Trends Genet. 1991;7:113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins N A, Copeland N G, Taylor B A, Lee B K. J Virol. 1982;43:26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh G, Kaur S, Stock J L, Jenkins N A, Gilbert D J, Copeland N G, Potter S S. Proc Natl Acad Sci USA. 1991;88:10706–10710. doi: 10.1073/pnas.88.23.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds A B, Jenkins N A, Gilbert D J, Copeland N G, Shapiro D N, Wu J, Daniel J M. Genomics. 1996;31:127–129. doi: 10.1006/geno.1996.0020. [DOI] [PubMed] [Google Scholar]
- 23.Tessarollo L, Tsoulfas P, Martin-Zanca D, Gilbert D J, Jenkins N A, Copeland N G, Parada L F. Development. 1993;118:463–475. doi: 10.1242/dev.118.2.463. [DOI] [PubMed] [Google Scholar]
- 24.Rothnagel J A, Longley M A, Bundman D S, Naylor S L, Lalley P A, Jenkins N A, Gilbert D J, Copeland N G, Roop D R. Genomics. 1994;23:450–456. doi: 10.1006/geno.1994.1522. [DOI] [PubMed] [Google Scholar]
- 25.Kuo S S, Mellentin J D, Copeland N G, Gilbert D J, Jenkins N A, Cleary M L. Oncogene. 1991;6:961–968. [PubMed] [Google Scholar]
- 26.Sun, X. J., Wang, L.-M., Zhang, Y., Yenush, L., Pons, S., Burks, D., Myers, M. G., Glasheen, E., Copeland, N. G., Jenkins, N. A., Lane, W. S., Pierce, J. H. & White, M. F. (1997) Mol. Endocrinol., in press. [DOI] [PubMed]
- 27.Jenkins N A, Gilbert D J, Yamamoto R, Kilimann M W, Copeland N G. Genomics. 1995;28:363–365. doi: 10.1006/geno.1995.1161. [DOI] [PubMed] [Google Scholar]
- 28.Green E L. Genetics and Probability in Animal Breeding Experiments. New York: Oxford Univ. Press; 1981. pp. 77–113. [Google Scholar]
- 29.Gorman C M, Gies D R, McCray G. DNA Prot Eng Tech. 1990;2:3–10. [Google Scholar]
- 30.Evan G I, Lewis G K, Ramsay G, Bishop J M. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caras I W, Weddell G N, Davitz M A, Nussenzweig V, Martin D W. Science. 1987;238:1280–1283. doi: 10.1126/science.2446389. [DOI] [PubMed] [Google Scholar]
- 32.Cheng H-J, Nakamoto M, Bergemann A D, Flanagan J G. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 33.Berger J, Hauber J, Hauber R, Geiger R, Cullen B. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- 34.Altschul S F, Gish W, Miller W, Myers E W, Lippman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 35.Lennon G G, Auffrey C, Polymeropoulos M, Soares M B. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- 36.Shirozu M, Tada H, Tashiro K, Nakamura T, Lopez N D, Nazarea M, Hamada T, Sato T, Nakano T, Honjo T. Genomics. 1996;37:273–280. doi: 10.1006/geno.1996.0560. [DOI] [PubMed] [Google Scholar]
- 37.Hoang B, Moos M, Vukicevic S, Luyten F P. J Biol Chem. 1996;271:26131–26137. doi: 10.1074/jbc.271.42.26131. [DOI] [PubMed] [Google Scholar]