Abstract
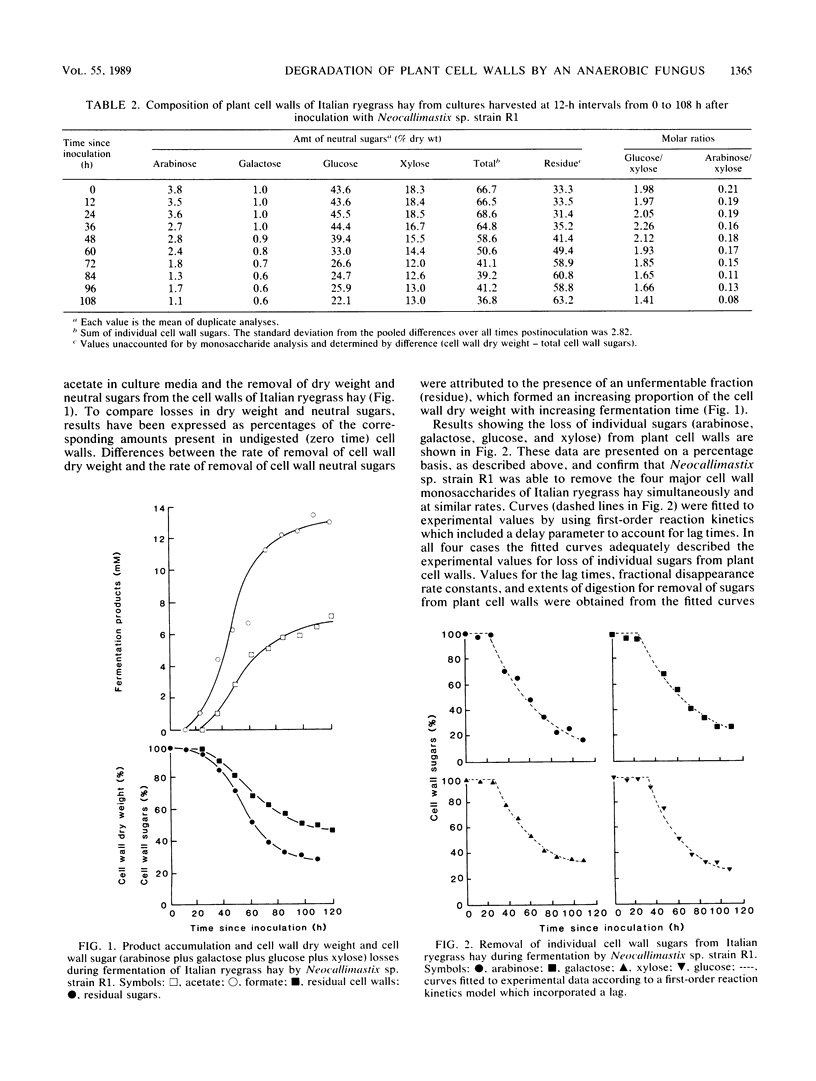
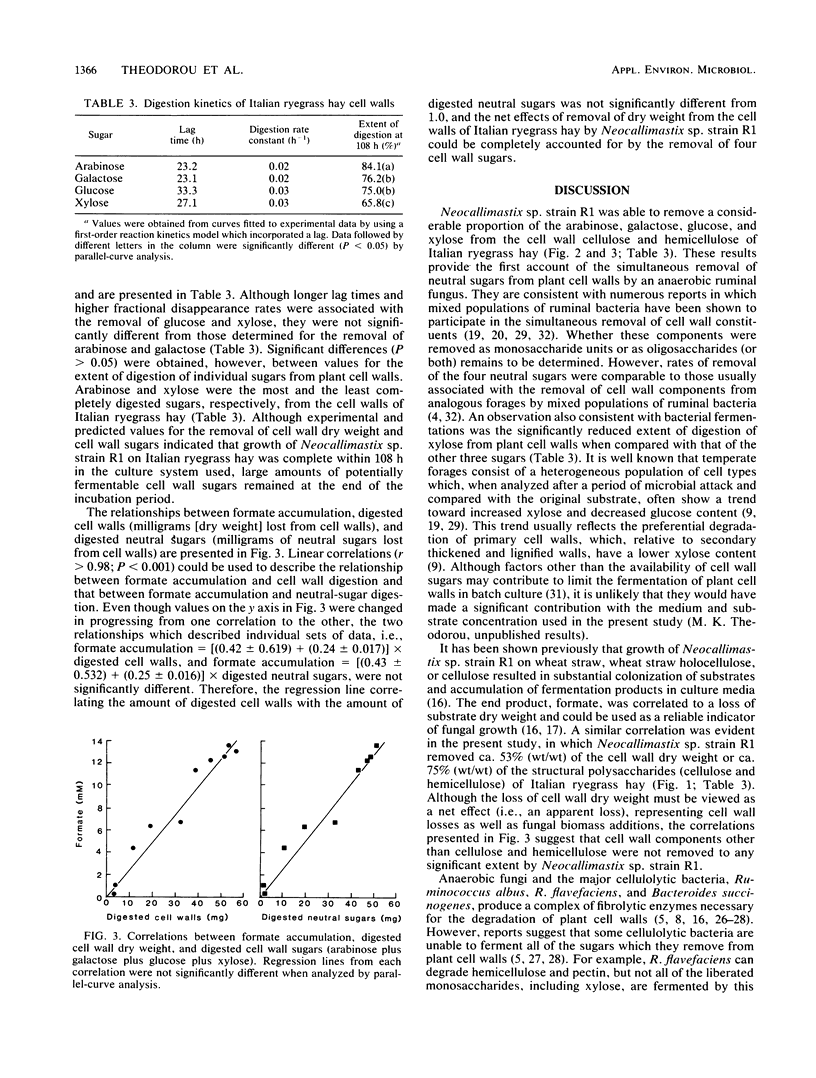
The anaerobic fungus Neocallimastix sp. strain R1 was grown for up to 5 days on a medium containing autoclaved Italian ryegrass hay as the carbon source. Culture supernatants and digested cell walls were harvested at 12-h intervals. Supernatants were analyzed for the fermentation products formate and acetate, and residual cell walls were analyzed for dry-matter and neutral-sugar losses. Fungal growth was accompanied by the digestion of plant cell walls and the accumulation of fermentation products in culture media. Dry-matter losses were accounted for by removal of four major neutral sugars (arabinose, galactose, glucose, and xylose) from the plant cell walls. First-order reaction kinetics could be used to describe the loss of each sugar. All cell wall sugars, including arabinose and galactose, which are not fermented by Neocallimastix sp. strain R1 were removed simultaneously. Although the rates of removal of individual sugars were similar, there were significant differences in their extents of removal: the extent of removal of arabinose exceeded that of the other three sugars, and xylose was the least digestible. This study provides the first account of simultaneous (nonpreferential) removal of neutral sugars from plant cell walls by an anaerobic fungus. Although in vitro techniques were used, these results indicate a potentially significant role for the anaerobic fungi as fiber digesters in the rumen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Rumen anaerobic fungi of cattle and sheep. Appl Environ Microbiol. 1979 Jul;38(1):148–158. doi: 10.1128/aem.38.1.148-158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. The rumen anaerobic fungi: colonizers of plant fibre. Ann Rech Vet. 1979;10(2-3):246–248. [PubMed] [Google Scholar]
- Dehority B. A. Mechanism of isolated hemicellulose and xylan degradation by cellulolytic rumen bacteria. Appl Microbiol. 1968 May;16(5):781–786. doi: 10.1128/am.16.5.781-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P. N., Wallace R. J. Microbial ecology and activities in the rumen: part 1. Crit Rev Microbiol. 1982 Apr;9(3):165–225. doi: 10.3109/10408418209104490. [DOI] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl Environ Microbiol. 1987 Jun;53(6):1216–1223. doi: 10.1128/aem.53.6.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Growth and fermentation of an anaerobic rumen fungus on various carbon sources and effect of temperature on development. Appl Environ Microbiol. 1987 Jun;53(6):1210–1215. doi: 10.1128/aem.53.6.1210-1215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A., Bauchop T. Fermentation of Cellulose to Methane and Carbon Dioxide by a Rumen Anaerobic Fungus in a Triculture with Methanobrevibacter sp. Strain RA1 and Methanosarcina barkeri. Appl Environ Microbiol. 1982 Jul;44(1):128–134. doi: 10.1128/aem.44.1.128-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpin C. G. Invasion of plant tissue in the rumen by the flagellate Neocallimastix frontalis. J Gen Microbiol. 1977 Feb;98(2):423–430. doi: 10.1099/00221287-98-2-423. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Isolation of cellulolytic phycomycete fungi from the caecum of the horse. J Gen Microbiol. 1981 Apr;123(2):287–296. doi: 10.1099/00221287-123-2-287. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol. 1975 Dec;91(2):249–262. doi: 10.1099/00221287-91-2-249. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Sphaeromonas communis. J Gen Microbiol. 1976 Jun;94(2):270–280. doi: 10.1099/00221287-94-2-270. [DOI] [PubMed] [Google Scholar]
- Theodorou M. K., Lowe S. E., Trinci A. P. The fermentative characteristics of anaerobic rumen fungi. Biosystems. 1988;21(3-4):371–376. doi: 10.1016/0303-2647(88)90035-4. [DOI] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Polysaccharide-degrading enzymes formed by three species of anaerobic rumen fungi grown on a range of carbohydrate substrates. Can J Microbiol. 1987 May;33(5):418–426. doi: 10.1139/m87-071. [DOI] [PubMed] [Google Scholar]


