Abstract
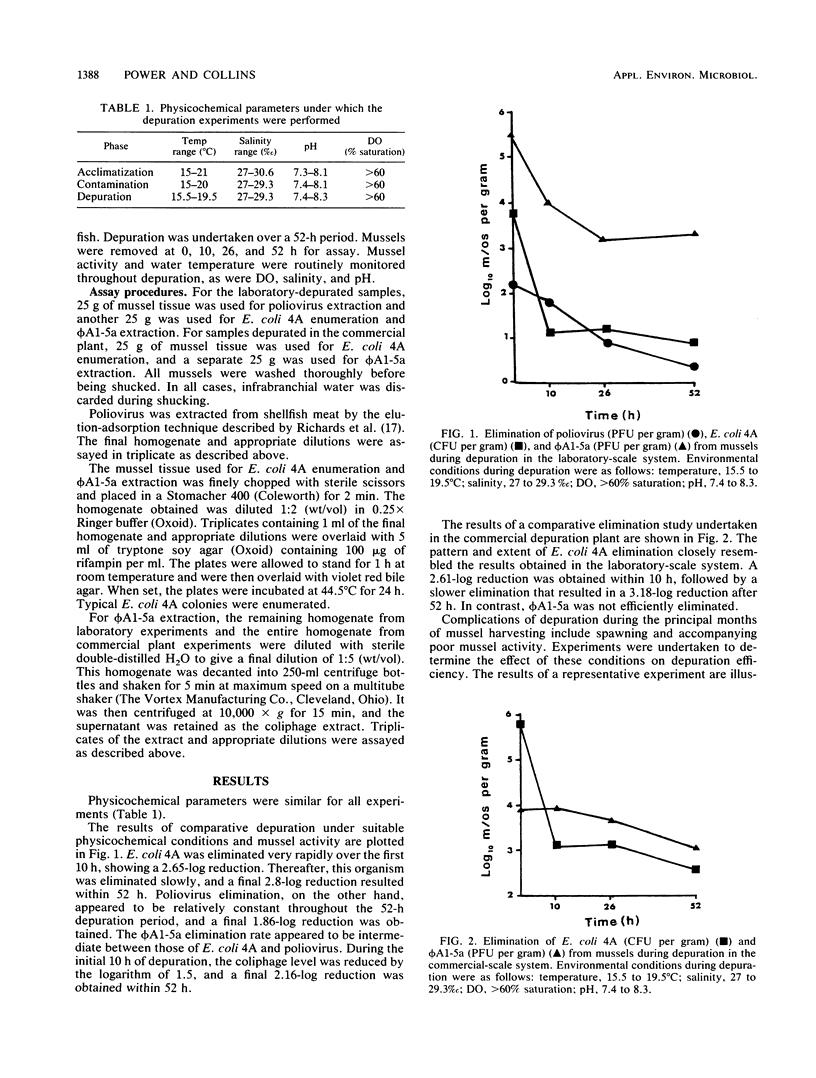
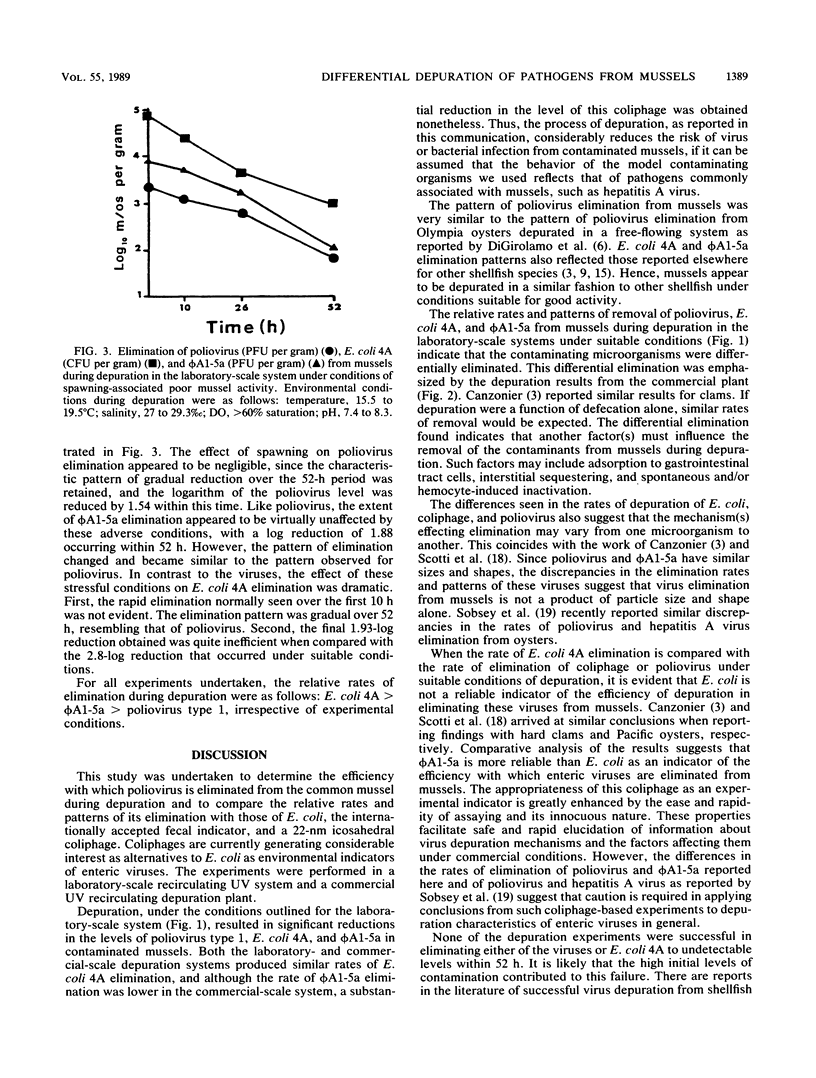
The elimination of sewage effluent-associated poliovirus, Escherichia coli, and a 22-nm icosahedral coliphage by the common mussel, Mytilus edulis, was studied. Both laboratory-and commercial-scale recirculating, UV depuration systems were used in this study. In the laboratory system, the logarithms of the poliovirus, E. coli, and coliphage levels were reduced by 1.86, 2.9, and 2.16, respectively, within 52 h of depuration. The relative patterns and rates of elimination of the three organisms suggest that they are eliminated from mussels by different mechanisms during depuration under suitable conditions. Poliovirus was not included in experiments undertaken in the commercial-scale depuration system. The differences in the relative rates and patterns of elimination were maintained for E. coli and coliphage in this system, with the logarithm of the E. coli levels being reduced by 3.18 and the logarithm of the coliphage levels being reduced by 0.87. The results from both depuration systems suggest that E. coli is an inappropriate indicator of the efficiency of virus elimination during depuration. The coliphage used appears to be a more representative indicator. Depuration under stressful conditions appeared to have a negligible affect on poliovirus and coliphage elimination rates from mussels. However, the rate and pattern of E. coli elimination were dramatically affected by these conditions. Therefore, monitoring E. coli counts might prove useful in ensuring that mussels are functioning well during depuration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canzonier W. J. Accumulation and elimination of coliphage S-13 by the hard clam, Mercenaria mercenaria. Appl Microbiol. 1971 Jun;21(6):1024–1031. doi: 10.1128/am.21.6.1024-1031.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo R., Liston J., Matches J. Uptake and elimination of poliovirus by West Coast oysters. Appl Microbiol. 1975 Feb;29(2):260–264. doi: 10.1128/am.29.2.260-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill O. N., Cubitt W. D., McSwiggan D. A., Watney B. M., Bartlett C. L. Epidemic of gastroenteritis caused by oysters contaminated with small round structured viruses. Br Med J (Clin Res Ed) 1983 Nov 19;287(6404):1532–1534. doi: 10.1136/bmj.287.6404.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann G. S., Murphy A. M., Christopher P. J., Auty E., Greenberg H. B. Norwalk virus gastroenteritis in volunteers consuming depurated oysters. Aust J Exp Biol Med Sci. 1981 Apr;59(Pt 2):219–228. doi: 10.1038/icb.1981.17. [DOI] [PubMed] [Google Scholar]
- Hoff J. C., Becker R. C. The accumulation and elimination of crude and clarified poliovirus suxpensions by shellfish. Am J Epidemiol. 1969 Jul;90(1):53–61. doi: 10.1093/oxfordjournals.aje.a121049. [DOI] [PubMed] [Google Scholar]
- Liu O. C., Seraichekas H. R., Murphy B. L. Viral depuration of the Northern quahaug. Appl Microbiol. 1967 Mar;15(2):307–315. doi: 10.1128/am.15.2.307-315.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. S., Collins J. K. Effects of cell culture and laboratory conditions on type 2 dengue virus infectivity. J Clin Microbiol. 1979 Aug;10(2):235–239. doi: 10.1128/jcm.10.2.235-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. R., Presnell M. W., Akin E. W., Cummins J. M., Liu O. C. Accumulation and elimination of poliovirus by the eastern oyster. Am J Epidemiol. 1966 Jul;84(1):40–50. doi: 10.1093/oxfordjournals.aje.a120626. [DOI] [PubMed] [Google Scholar]
- Richards G. P., Goldmintz D., Green D. L., Babinchak J. A. Rapid methods for extraction and concentration of poliovirus from oyster tissues. J Virol Methods. 1982 Dec;5(5-6):285–291. doi: 10.1016/0166-0934(82)90019-2. [DOI] [PubMed] [Google Scholar]



