Abstract
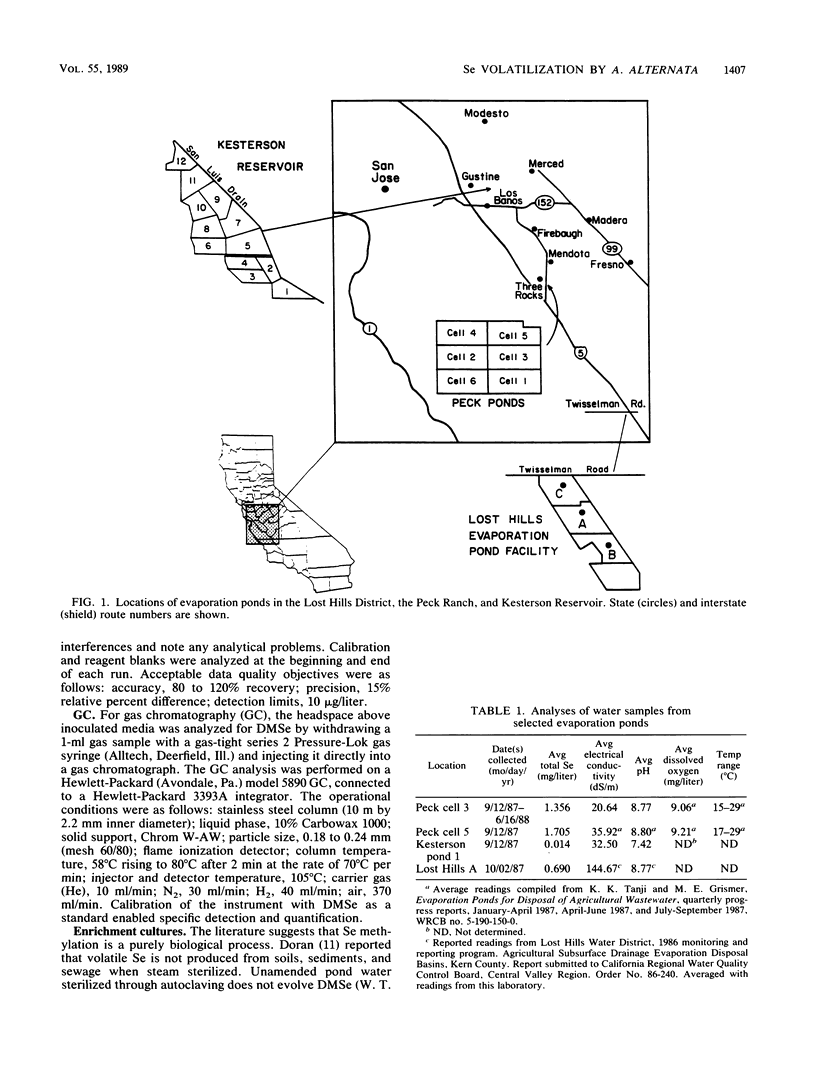
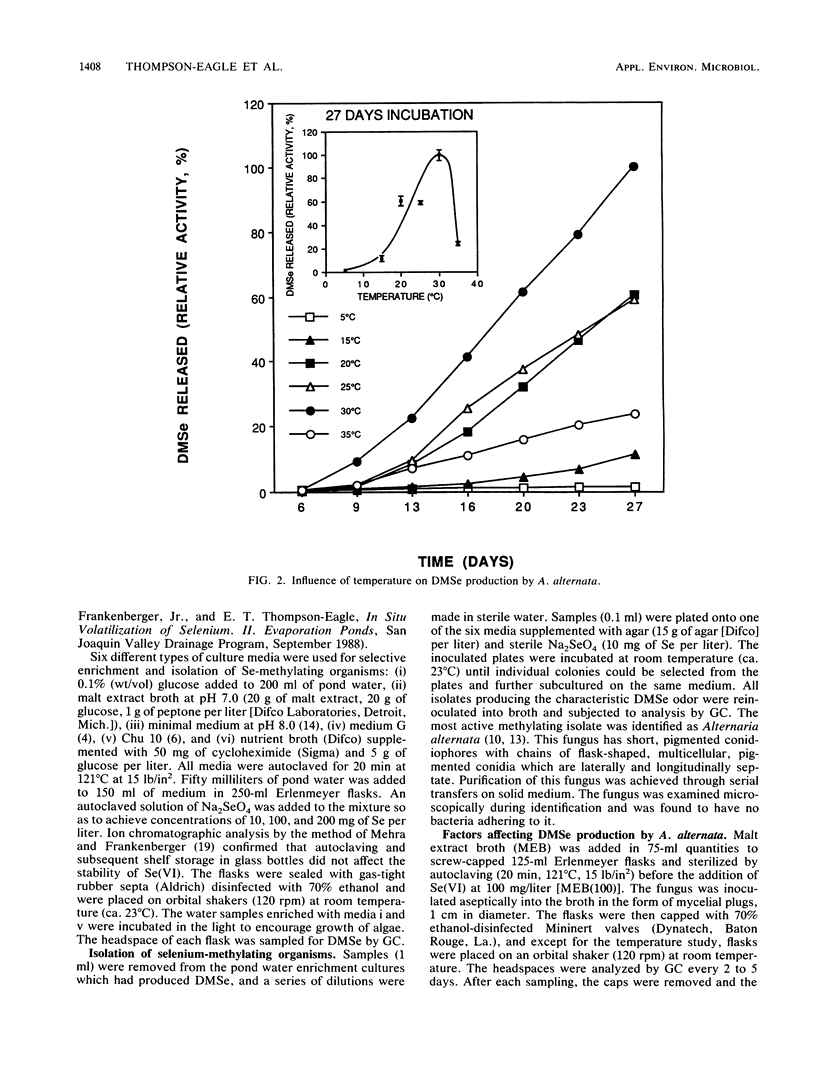
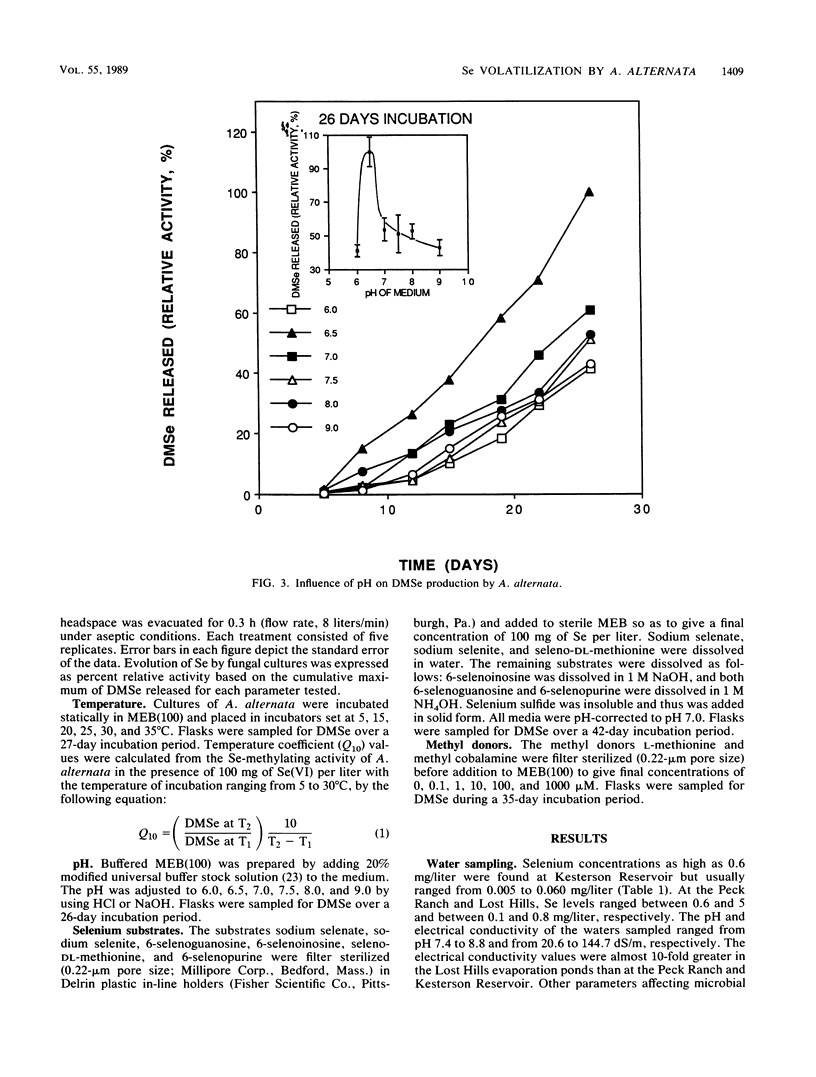
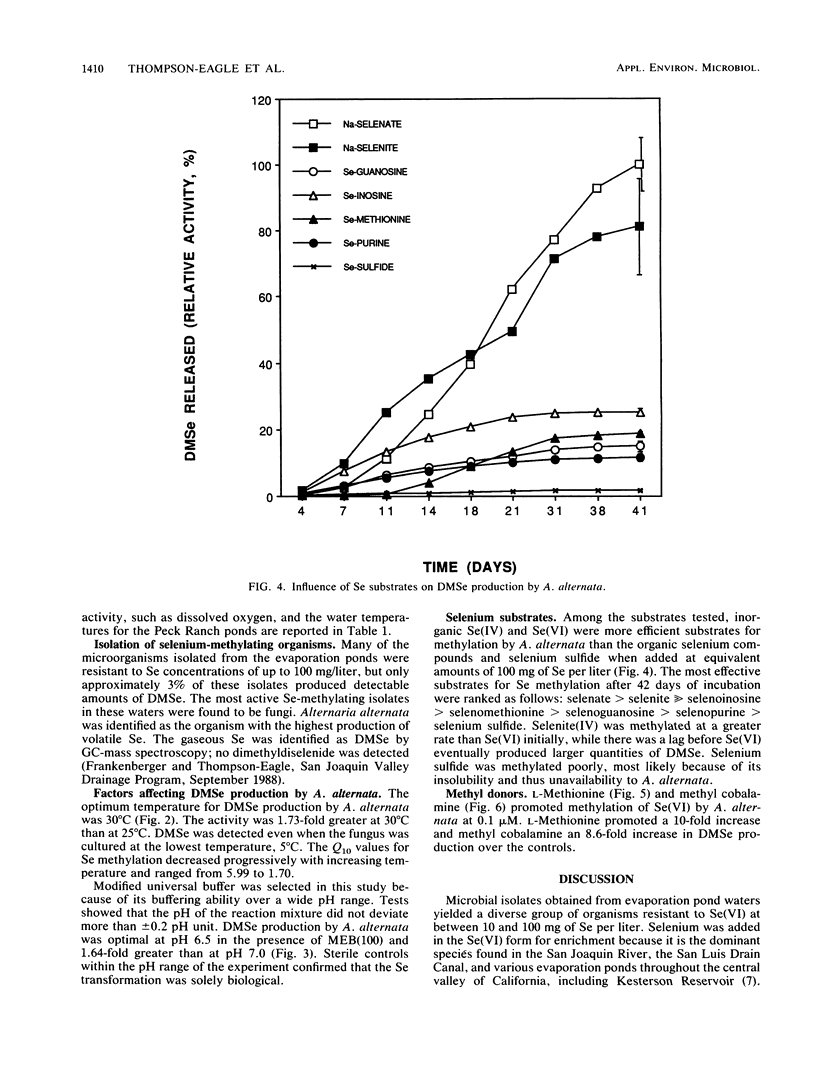
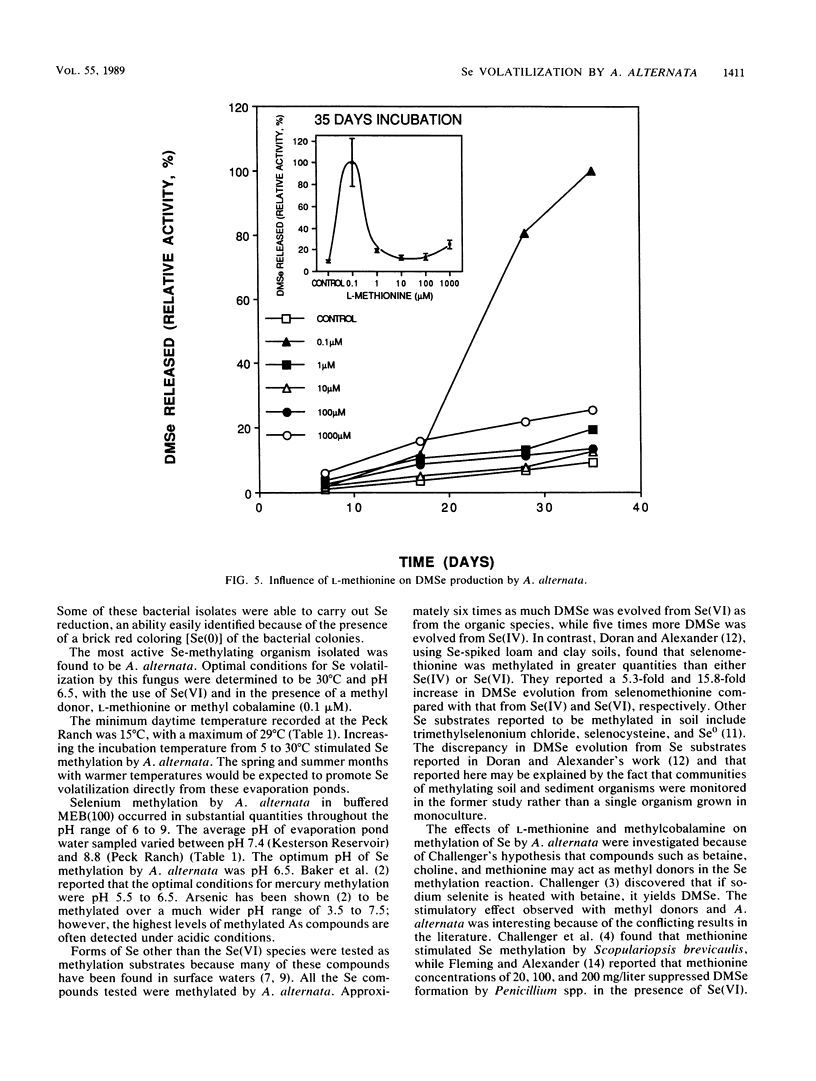
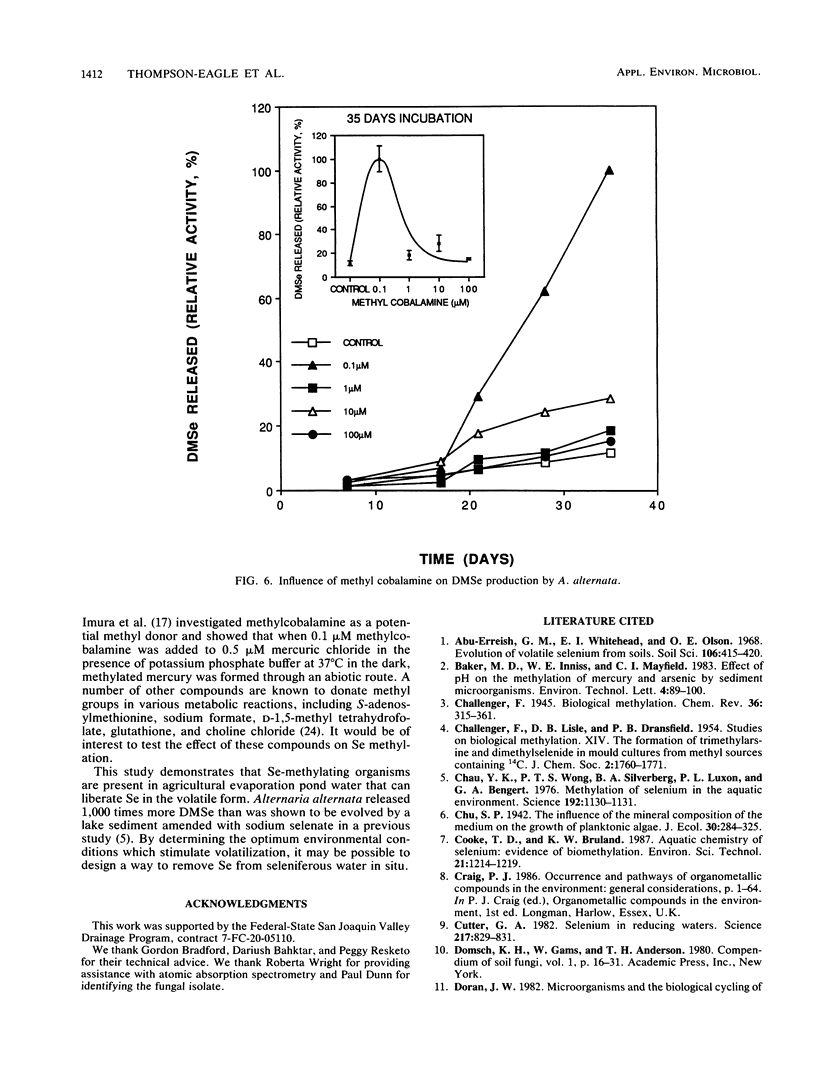
Seleniferous water continues to be a serious problem to wildlife in the central valley of California. Water samples collected from Kesterson Reservoir, Peck Ranch, and Lost Hills evaporation pond facilities contained between 0.005 and 5 mg of Se per liter. The objective of this study was to isolate Se-methylating organisms in evaporation pond water and to assess, through enrichment and manipulation of their optimal growth parameters, the environmental factors which govern microbial Se methylation. Alternaria alternata was isolated as an active Se-methylating organism. The volatile product was identified as dimethylselenide. The effects of pH, temperature, Se substrates, and methyl donors on the ability of A. alternata to methylate Se were investigated in liquid medium containing 100 mg of Se per liter. The optimum pH and temperature for methylation were 6.5 and 30°C, respectively. Selenate and selenite were methylated more rapidly than selenium sulfide and various organic Se compounds (6-selenoguanosine, 6-selenoinosine, seleno-dl-methionine, and 6-selenopurine). l-Methionine and methyl cobalamine (0.1 μM) stimulated dimethylselenide production. This study demonstrates that Se-methylating organisms are present in evaporation pond water and are capable of liberating substantial quantities of Se in the volatile dimethylselenide form. By determining the optimum environmental conditions which stimulate volatilization, it may be possible to design a way to remove Se from seleniferous water in situ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chau Y. K., Wong P. T., Silverberg B. A., Luxon P. L., Bengert G. A. Methylation of selenium in the aquatic environment. Science. 1976 Jun 11;192(4244):1130–1131. doi: 10.1126/science.192.4244.1130. [DOI] [PubMed] [Google Scholar]
- Cutter G. A. Selenium in reducing waters. Science. 1982 Aug 27;217(4562):829–831. doi: 10.1126/science.217.4562.829. [DOI] [PubMed] [Google Scholar]
- Fleming R. W., Alexander M. Dimethylselenide and dimethyltelluride formation by a strain of Penicillium. Appl Microbiol. 1972 Sep;24(3):424–429. doi: 10.1128/am.24.3.424-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganther H. E., Levander O. A., Baumann C. A. Dietary control of selenium volatilization in the rat. J Nutr. 1966 Jan;88(1):55–60. doi: 10.1093/jn/88.1.55. [DOI] [PubMed] [Google Scholar]
- Imura N., Sukegawa E., Pan S. K., Nagao K., Kim J. Y., Kwan T., Ukita T. Chemical methylation of inorganic mercury with methylcobalamin, a vitamin B12 analog. Science. 1971 Jun 18;172(3989):1248–1249. doi: 10.1126/science.172.3989.1248. [DOI] [PubMed] [Google Scholar]
- McCONNELL K. P., PORTMAN O. W. Toxicity of dimethyl selenide in the rat and mouse. Proc Soc Exp Biol Med. 1952 Feb;79(2):230–231. doi: 10.3181/00379727-79-19333. [DOI] [PubMed] [Google Scholar]
- Wilber C. G. Toxicology of selenium: a review. Clin Toxicol. 1980 Sep;17(2):171–230. doi: 10.3109/15563658008985076. [DOI] [PubMed] [Google Scholar]
- ZALOKAR M. Reduction of selenite by Neurospora. Arch Biochem Biophys. 1953 Jun;44(2):330–337. doi: 10.1016/0003-9861(53)90051-4. [DOI] [PubMed] [Google Scholar]



