Abstract
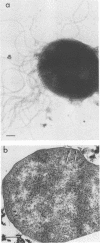
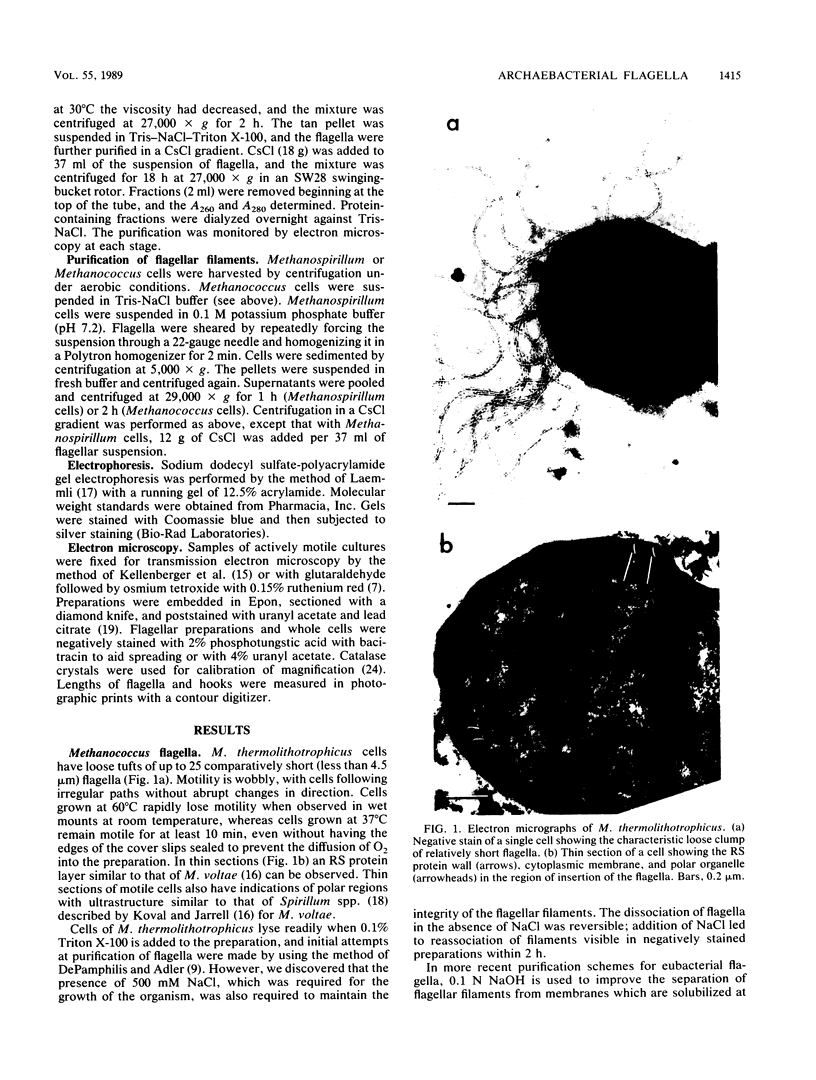
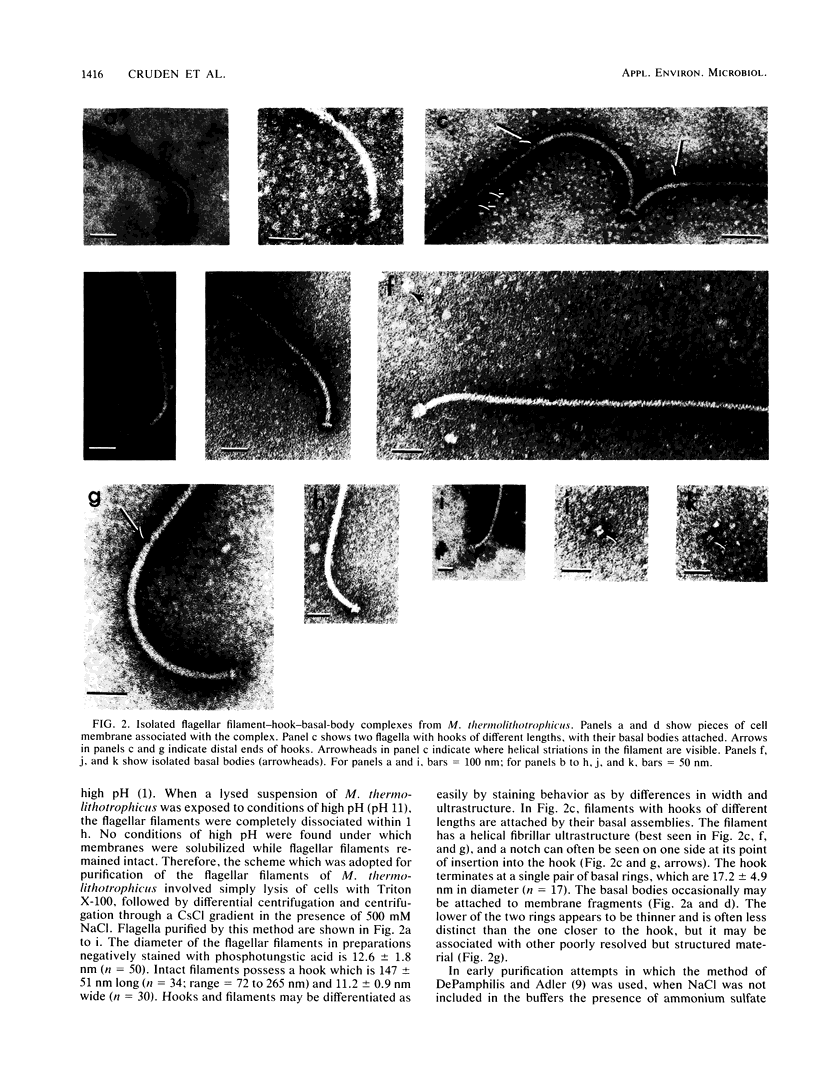
The flagella of the archaebacteria Methanococcus thermolithotrophicus and Methanospirillum hungatei enter the cells in regions with ultrastructure resembling that of the polar organelles found in a variety of eubacteria. Flagella of both organisms consist of a filament, a hook, and a basal body with two rings similar to those of gram-positive eubacteria. The integrity of the flagella of M. thermolithotrophicus is lost in the absence of high salt concentrations, and those of both organisms are unstable at high pH. The flagellar filaments of M. hungatei are composed of two flagellins of 24 and 26 kilodaltons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa S. I., Dean G. E., Jones C. J., Macnab R. M., Yamaguchi S. Purification and characterization of the flagellar hook-basal body complex of Salmonella typhimurium. J Bacteriol. 1985 Mar;161(3):836–849. doi: 10.1128/jb.161.3.836-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Oesterhelt D. Morphology, function and isolation of halobacterial flagella. J Mol Biol. 1984 Jul 15;176(4):459–475. doi: 10.1016/0022-2836(84)90172-4. [DOI] [PubMed] [Google Scholar]
- Alam M., Oesterhelt D. Purification, reconstitution and polymorphic transition of halobacterial flagella. J Mol Biol. 1987 Apr 5;194(3):495–499. doi: 10.1016/0022-2836(87)90677-2. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Stewart M., Doyle R. J., Sprott G. D. Unusual stability of the Methanospirillum hungatei sheath. J Bacteriol. 1985 May;162(2):728–737. doi: 10.1128/jb.162.2.728-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Belay N., Rajagopal B. S. Assimilatory reduction of sulfate and sulfite by methanogenic bacteria. Appl Environ Microbiol. 1986 Apr;51(4):703–709. doi: 10.1128/aem.51.4.703-709.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmokoff M. L., Jarrell K. F., Koval S. F. Isolation of flagella from the archaebacterium Methanococcus voltae by phase separation with Triton X-114. J Bacteriol. 1988 Apr;170(4):1752–1758. doi: 10.1128/jb.170.4.1752-1758.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval S. F., Jarrell K. F. Ultrastructure and biochemistry of the cell wall of Methanococcus voltae. J Bacteriol. 1987 Mar;169(3):1298–1306. doi: 10.1128/jb.169.3.1298-1306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. J., Hills G. J., Henwood J. A., Harris J. E., Archer D. B. Three-dimensional architecture of the cell sheath and septa of Methanospirillum hungatei. J Bacteriol. 1985 Feb;161(2):750–757. doi: 10.1128/jb.161.2.750-757.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauschel H. D. ATPase and cytochrome oxidase activities at the polar organelle in swarm cells of Sphaerotilus natans: an ultrastructural study. Arch Microbiol. 1985 May;141(4):303–308. doi: 10.1007/BF00428841. [DOI] [PubMed] [Google Scholar]
- Wieland F., Paul G., Sumper M. Halobacterial flagellins are sulfated glycoproteins. J Biol Chem. 1985 Dec 5;260(28):15180–15185. [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]