Abstract
In a previous study, we demonstrated that sodium salicylate (NaSal) selectively inhibits tumor necrosis factor (TNF)-induced activation of the p42 and p44 mitogen-activated protein kinases (MAPKs) (known as extracellular signal-regulated kinases). Here we show that in normal human FS-4 fibroblasts NaSal inhibits TNF-induced activation of another member of the MAPK family, the c-Jun N-terminal kinase/stress-activated protein kinase. c-Jun N-terminal kinase activation induced by interleukin 1 or epidermal growth factor was less strongly inhibited by NaSal. Unexpectedly, treatment of FS-4 cells with NaSal alone produced a strong activation of p38 MAPK and cell death by apoptosis. NaSal-induced apoptosis was blocked by the selective p38 MAPK inhibitor SB-203580, indicating that p38 MAPK serves as a mediator of NaSal-induced apoptosis in human fibroblasts. Activation of p38 MAPK and the resulting induction of apoptosis may be important in the demonstrated antineoplastic actions of nonsteroidal anti-inflammatory drugs.
Keywords: signal transduction, cytokines, nonsteroidal anti-inflammatory drugs
Three structurally related mitogen-activated protein kinase (MAPK) subfamilies have been identified in mammalian cells: the p42 and p44 (extracellular signal-regulated kinase; ERK) kinases, the c-Jun N-terminal kinases (JNK)/stress-activated protein kinases, and the p38 kinase (1–3). All three MAPK subfamilies phosphorylate substrates on serine and threonine residues located adjacent to proline residues, and members of all MAPKs are activated as a result of simultaneous phosphorylation on threonine and tyrosine residues by upstream dual-specificity kinases. However, the three MAPK subfamilies are activated in response to different extracellular stimuli, have different downstream targets and, therefore, perform different functions. ERKs are characteristically activated by growth factors, usually by means of a Ras-Raf-1-dependent cascade (1–3), whereas JNK (4–6) and p38 kinase (7–9) are strongly activated by UV irradiation, osmotic stress, and the inflammatory cytokines tumor necrosis factor (TNF) and interleukin 1 (IL-1). However, the specificity of activating stimuli is not absolute, as both TNF and IL-1 activate ERKs in some cell lines (10–12) and growth factors [e.g., epidermal growth factor (EGF)] weakly activate JNK and p38 kinase (4, 5, 8).
While TNF treatment of cells leads to the activation of all three MAPK subfamilies, there is limited information concerning the roles of the MAPKs in TNF actions. Several studies have demonstrated the significant role of JNK in TNF and IL-1 signaling, mediating the activation of transcription factors such as c-Jun, and the activation of genes such as c-fos (6, 13). In a recent study, Beyaert et al. (14) found that the p38 kinase inhibitor SB-203580 suppressed the induction of some cellular genes by TNF, but did not affect the cytotoxicity of TNF in murine L929 cells. In other cells, apoptosis induced by ceramide or by TNF was shown to require the function of JNK and its target, c-Jun (15). Earlier, Xia et al. (16) demonstrated that activation of JNK and p38 kinase with concurrent inhibition of ERK activity is critical for induction of apoptosis triggered by the withdrawal of nerve growth factor in rat PC-12 cells.
Sodium salicylate (NaSal) and aspirin were recently shown to inhibit activation of the transcription factor NF-κB by TNF and other agents (17), and this inhibition was attributed to the ability of NaSal to prevent phosphorylation and subsequent degradation of the inhibitor IκB-α (17, 18). We showed that NaSal inhibited TNF-induced ERK activation but did not affect ERK activation by EGF under the same conditions, indicating that NaSal selectively interfered with a TNF-activated pathway (19). In the present study we show that NaSal also inhibits TNF-induced activation of JNK. In addition, we demonstrate that treatment with NaSal alone produces a strong activation of p38 kinase as well as cell death by apoptosis, and that the p38 kinase inhibitor SB-203580 prevents NaSal-induced apoptosis. The ability of NaSal and some other nonsteroidal anti-inflammatory drugs (NSAIDs) to induce apoptosis has been linked to the documented antineoplastic actions of these drugs (20–22). Our demonstration that p38 kinase activity is essential for NaSal-induced apoptosis thus suggests a role for p38 kinase in the antineoplastic actions of NSAIDs.
MATERIALS AND METHODS
Cell Culture.
Normal human diploid FS-4 fibroblasts (11, 19, 23) were cultured in Eagle’s minimal essential medium (MEM) supplemented with 5% heat-inactivated fetal bovine serum. Before use in experiments, FS-4 cells were serum-starved for 2–3 days in MEM with 0.25% fetal bovine serum. COS-1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum.
Kinase Assays.
Kinase assays were performed essentially as described (6) with some modifications. Briefly, whole cell lysates were generated using a buffer consisting of 1% Nonidet P-40, 50 mM Hepes (pH 7.5), 100 mM NaCl, 2 mM EDTA, 1 mM pyrophosphate, 10 mM sodium orthovanadate, 3 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, and 100 mM sodium fluoride. To assay JNK activity, lysates were incubated for 1 h at 4°C with glutathione S-transferase (GST)-c-Jun (containing amino acids 1–223) coupled to glutathione-agarose beads (24). The beads were then washed three times with lysis buffer, and twice with kinase buffer (20 mM Hepes, pH 7.6/20 mM MgCl2/20 mM β-glycerophosphate/10 mM sodium fluoride/10 μM ATP/0.2 mM DTT/0.2 mM sodium orthovanadate). After a 30-min incubation at 30°C in kinase buffer containing 15 μCi (1 Ci = 37 GBq) of [γ-32P]ATP, the kinase reaction was terminated by the addition of protein sample buffer. Phosphorylated reaction products were visualized following SDS/PAGE and autoradiography. Alternatively, an antibody to JNK1 (C17; Santa Cruz Biotechnology) and protein G-agarose were used to form an immunoprecipitate from the same lysates that was then used in the kinase assay with GST-c-Jun. According to the manufacturer, the C17 rabbit polyclonal antibody is reactive with isoforms of JNK1 and, to a lesser extent, with isoforms of JNK2. For the assay of p38 MAPK activity, COS-1 cells were transfected with 5 μg of the epitope-tagged pCMV-Flag-p38 MAPK construct (8) using a calcium phosphate mammalian cell transfection kit (5′ → 3′). Cells were then serum-starved in DMEM/0.5% fetal bovine serum for 18 h, treated as indicated, and lysed. Epitope-tagged p38 MAPK was immunoprecipitated from the lysates with the M2 monoclonal antibody to Flag (IBI-Kodak) bound to protein G-agarose, and subsequently used in a kinase assay with GST-ATF2 as substrate, essentially as described above for the kinase assay with GST-c-Jun as substrate.
Northern Blot Analysis.
Aliquots of total cytoplasmic RNA (10 μg) were isolated (25), electrophoresed on Mops/1% agarose gels and then blotted onto a Nytran Plus membrane (Schleicher & Schuell). Blots were prehybridized and hybridized with QuikHyb solution (Stratagene). The c-fos and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs (26) were labeled by random priming using [α-32P]dCTP and a Rediprime labeling kit (Amersham). The GAPDH probe served as an internal control for RNA loading and transfer.
Immunoblotting.
Western blot analysis was performed as described (19). The anti-phosphotyrosine antibody, used at a 1:200 dilution, was obtained from J. Schlessinger (New York University Medical Center). Both the anti-phospho-p38 and anti-p38 MAPK antibodies (New England Biolabs) were used at a 1:1000 dilution. Antibody–antigen complexes were detected with the aid of horseradish peroxidase-conjugated staphylococcal protein A (Life Technologies, Grand Island, NY), and a chemiluminescent substrate development kit (Kirkegaard & Perry Laboratories).
Apoptosis and Its Inhibition by SB-203580.
FS-4 cells were plated on glass coverslips, serum-starved, treated with NaSal, washed with PBS, and then fixed with a 4% paraformaldehyde solution. Cells were then permeabilized with PBS/0.5% Triton X-100, and nuclei were stained for 20 min with the chromatin-staining Hoechst 33342 dye (Sigma). The coverslips were then washed, mounted onto slides, and viewed with a fluorescence microscope. The p38 MAPK inhibitor SB-203580 (9, 27–29) was obtained from John C. Lee (SmithKline Beecham). SB-203580 was solubilized in dimethyl sulfoxide. Control experiments demonstrated that treatment with the same concentration of dimethyl sulfoxide alone had no effect either on FS-4 cell viability or on the cytotoxicity of NaSal for FS-4 cells.
RESULTS
NaSal Inhibits TNF-Induced JNK Activation.
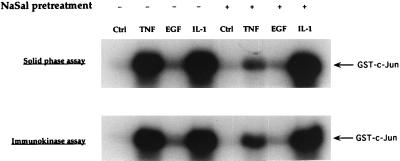
FS-4 cultures were either treated for 1 h with NaSal or left untreated, and then stimulated with TNF, EGF, or IL-1. To determine the levels of JNK activity, cell lysates were analyzed for their ability to phosphorylate c-Jun protein in an in vitro solid-phase kinase assay (Fig. 1 Upper). To ascertain that the kinase activity detected in this assay is indeed mediated by JNK, the same assay was also performed with cell lysates from which, prior to the kinase assay, JNK was immunoprecipitated with a specific antibody (Fig. 1 Lower). The two types of assay yielded identical results, showing a marked stimulation of kinase activity by TNF and IL-1, and weak stimulation by EGF. Treatment with NaSal had a potent inhibitory effect on TNF-induced kinase activity, but was much less effective in reducing EGF- and IL-1-induced JNK activity. NaSal was consistently less effective in inhibiting IL-1-induced JNK activity also when suboptimal IL-1 or TNF doses were used (not shown). The finding that NaSal more strongly inhibited JNK activation by TNF than by IL-1 is surprising because these two cytokines are similar in many of their actions and they activate similar signaling pathways (30, 31).
Figure 1.
Inhibition of TNF-induced JNK activation by NaSal. Serum-starved FS-4 cells (19) were treated for 1 h with 20 mM NaSal. They were then either left untreated (Ctrl) or treated for 15 min with TNF (20 ng/ml), EGF (30 ng/ml), or IL-1-α (4 ng/ml). Lysates were generated, and used directly in a protein kinase assay with GST-c-Jun (solid-phase assay, Upper). An antibody to JNK1 and protein G-agarose were used to form an immunoprecipitate from the same lysates that then was used in the kinase assay with GST-c-Jun (immunokinase assay, Lower). Phosphorylation reactions were initiated by the addition of [γ-32P]ATP and terminated by the addition of protein sample buffer. The reaction mixtures were separated by SDS/PAGE, and phosphorylated GST-c-Jun protein was visualized by autoradiography.
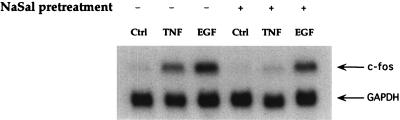
ERK and JNK MAPKs phosphorylate members of the ternary complex factor family (TCF/Elk-1), which results in the transcriptional activation of c-fos (13, 32–34). Therefore, we examined the effect of NaSal on the induction of c-fos mRNA by TNF or EGF. As previously reported (25), both TNF and EGF enhanced c-fos mRNA levels. NaSal inhibited c-fos mRNA induction by TNF, whereas induction by EGF was only modestly reduced (Fig. 2).
Figure 2.
Inhibition of TNF-induced c-fos mRNA induction by NaSal. Serum-starved FS-4 cells were treated for 1 h with 20 mM NaSal. They were then left untreated (Ctrl) or treated for 30 min with either TNF (20 ng/ml) or EGF (30 ng/ml). Total cellular RNA was subjected to Northern analysis. The blot was hybridized with 32P-labeled c-fos and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probes.
NaSal Induces p38 Kinase Activation.
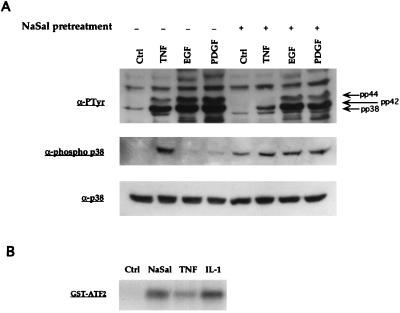
We then attempted to determine whether NaSal also inhibits TNF-induced activation of another member of the MAPK family, the p38 kinase (7–9). Cultures of FS-4 cells were first treated with NaSal or left untreated, and then stimulated with TNF, EGF, or platelet-derived growth factor (PDGF). Cell lysates were prepared and phosphotyrosine-containing bands were visualized by immunoblot analysis (Fig. 3A, Top). As previously shown (10–12), treatment with TNF, as well as treatment with EGF or PDGF, led to an increase in tyrosine phosphorylation of ≈44- and ≈42-kDa bands, corresponding to the two known forms of ERK MAPKs (pp44 and pp42) (19). NaSal treatment inhibited the constitutively present pp42 band in unstimulated cultures and the up-regulation of the pp44 and pp42 bands in TNF-treated cultures, while having a less pronounced inhibitory effect on the EGF- and PDGF-induced pp44 and pp42 bands. In addition, a phosphotyrosine-containing band migrating immediately below the p42 band (labeled “pp38”) was detected after treatment with TNF, but not upon stimulation with EGF or PDGF. The position of this band, along with the fact that it was induced by TNF but not by EGF or PDGF, suggested that it corresponds to p38 MAPK. Unexpectedly, cultures treated with NaSal alone, as well as those treated with NaSal and then stimulated with TNF, EGF, or PDGF, all showed the presence of this ≈38-kDa phosphotyrosine-containing band. To confirm that the band labeled “pp38” indeed represents the p38 kinase, the same cell lysates were immunoblotted with an antibody that specifically recognizes phosphotyrosine residues on p38 MAPK (Fig. 3A Middle). The latter analysis confirmed that tyrosine phosphorylation of the p38 kinase was enhanced not only by TNF but also by treatment with NaSal. The total amounts of p38 protein were not affected by these treatments (Fig. 3A Bottom).
Figure 3.
Activation of p38 MAPK by NaSal. (A) Western blot analyses of lysates generated from FS-4 cells. Serum-starved cells were treated for 1 h with 20 mM NaSal. They were then either left untreated (Ctrl) or treated for 15 min with TNF (20 ng/ml), EGF (30 ng/ml), or PDGFbb (50 ng/ml). Lysates were blotted with antibodies against phosphotyrosine (α-PTyr, Top), antibodies specific for the tyrosine-phosphorylated form of p38 MAPK (Middle), or antibodies to p38 MAPK protein (Bottom). Arrows in Top denote positions of the phosphorylated MAPKs pp38, pp42, and pp44. (B) Assay of p38 kinase activity. COS-1 cells were transfected with an epitope-tagged p38 MAPK expression vector (pCMV-Flag-p38 MAPK; ref. 8), serum-starved for 16 h, and then left untreated (Ctrl) or stimulated for 15 min with NaSal (20 mM), TNF (20 ng/ml), or IL-1 (4 ng/ml). Flag-p38 MAPK was then immunoprecipitated, and kinase activity was assayed by incubating the immunoprecipitates with [γ-32P]ATP and GST-ATF2 as substrate. The reaction mixtures were separated by SDS/PAGE, and phosphorylated GST-ATF2 was visualized by autoradiography. Equal expression of Flag-p38 MAPK in each lysate prior to immunoprecipitation was confirmed by Western blot analysis (data not shown).
To determine directly whether p38 kinase activity is stimulated by NaSal, COS cells were transfected with an expression plasmid encoding an epitope-tagged p38 kinase protein (8) and then treated with NaSal, TNF, or IL-1. p38 kinase activity was determined in an immune complex kinase assay using GST-ATF2 protein as a substrate (8). This specific p38 kinase assay confirmed that NaSal, IL-1 and, to a lesser extent, TNF increased p38 kinase activity in COS cells (Fig. 3B). The fact that TNF was less active in this assay than IL-1 may be due to the lower responsiveness of COS cells to TNF action.
p38 Kinase Activation by NaSal Leads to Apoptosis.
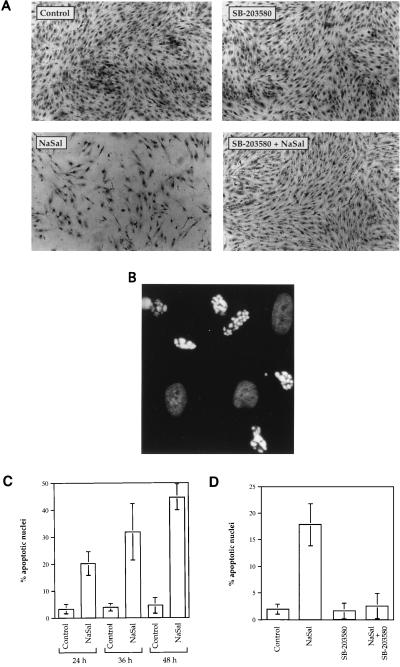
We observed that treatment of FS-4 cells with NaSal for longer time periods resulted in cytotoxicity. Since p38 kinase was earlier shown to contribute to the induction of programmed cell death in some cell systems (16, 35), we considered the possibility that p38 activation was responsible for the cytotoxic action of NaSal. To test this hypothesis we determined whether the highly specific p38 kinase inhibitor, the pyridinylimidazole compound SB-203580 (9, 27–29, 36), affected NaSal-induced cytotoxicity. Others have shown that at concentrations of up to 10 μM SB-203580 inhibited p38 kinase activity induced by TNF, IL-1, UV, or sorbitol, but not JNK activity induced by the same agents (36). We confirmed that 10 μM SB-203580 inhibited both TNF- and NaSal-induced p38 kinase activity in vitro, while not inhibiting TNF-induced JNK activity (data not shown). SB-203580, which by itself had no effect on cell morphology or viability, significantly reduced the cytotoxic action of NaSal (Fig. 4A). To determine whether NaSal induces cell death by apoptosis, FS-4 cells treated with NaSal or control cultures were stained with the Hoechst 33342 blue dye and analyzed for nuclear fragmentation. A large proportion of cell nuclei in the cultures treated with NaSal showed typical signs of apoptosis (Fig. 4B), while untreated cells did not (not shown). The ability of NaSal to induce apoptosis was confirmed by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling method (37) (data not shown). The proportion of cells undergoing apoptosis increased with the time of incubation with NaSal (Fig. 4C). Treatment of cells with NaSal and SB-203580 produced a strong reduction in the number of cells with apoptotic nuclei (Fig. 4D), demonstrating that the specific inhibitor of p38 kinase indeed suppressed NaSal-induced apoptosis.
Figure 4.
Induction of apoptosis by NaSal and its inhibition by SB-203580. (A) Cytotoxicity of NaSal in FS-4 cultures is inhibited by SB-203580. Serum-starved cells were either left untreated (Control), or treated for 48 h with SB-203580 (10 μM), NaSal (20 mM), or a combination of SB-203580 and NaSal at these same concentrations. For the combination treatment, cells were incubated with SB-203580 for 1.5 h before NaSal addition. Shown are photomicrographs of cells that were fixed and stained with naphthol-blue-black (23). (B) NaSal induces cell death by apoptosis. FS-4 cells were treated for 12 h with 20 mM NaSal, stained with Hoechst dye 33342, and examined by fluorescence microscopy. Photomicrograph demonstrates typical appearance of brightly staining highly vesiculated apoptotic nuclei with condensed chromatin. Also visible are less intensely stained nonapoptotic oval nuclei. (C) Time course of the induction of apoptosis by NaSal. Serum-starved FS-4 cells were either left untreated (Control) or treated with 20 mM NaSal for the indicated time periods. Hoechst dye-stained nuclei from at least six different microscopic fields were counted. Data shown are means ± SD from a representative experiment. Similar results were obtained in three other experiments. (D) Inhibition of NaSal-induced apoptosis by SB-203580. Serum-starved FS-4 cells were either left untreated (Control) or treated for 36 h with SB-203580 (10 μM), NaSal (20 mM), or a combination of SB-203580 and NaSal. For the combination treatment, cells were incubated with SB-203580 for 1.5 h before NaSal addition. Hoechst dye-stained nuclei from at least six different microscopic fields were counted. Data shown are means ± SD from a representative experiment. Similar results were obtained in three other experiments.
We also determined whether SB-203580 inhibited TNF-induced cytotoxicity. In many types of cells, including FS-4 fibroblasts, TNF is cytotoxic only in the presence of cycloheximide or other inhibitors of protein and RNA synthesis (38), whereas, in the absence of metabolic inhibitors TNF is mitogenic for FS-4 cells (23). Therefore, serum-starved confluent FS-4 cells were treated for 48 h with a combination of TNF (20 ng/ml) and cycloheximide (40 μg/ml), in the absence or presence of SB-203580 (10 μM). Under these conditions, treatment with either TNF alone or cycloheximide alone caused no significant cytotoxicity in FS-4 cultures within 48 h, whereas treatment with the two agents together resulted in ≈60% cell lysis. SB-203580 failed to protect cells from this cytotoxic action (data not shown), thus demonstrating that p38 kinase activity is not essential for TNF-mediated cytotoxicity in this system.
DISCUSSION
In an earlier study, we showed that NaSal inhibited TNF-induced ERK activation in normal human FS-4 fibroblasts, but did not affect ERK activation by EGF under the same conditions (19). ERK activation by IL-1 was also less strongly inhibited by NaSal than TNF-induced ERK activation (P.S., E.Y.S., and J.V., unpublished data). Our present results show that NaSal affects differentially the activation of the three known MAPK subfamilies; it inhibits TNF-induced ERK and JNK activation, while inducing p38 kinase activation. As the same dose of NaSal was either ineffective or much less effective in inhibiting ERK and JNK activation by other cytokines or growth factors, including EGF, PDGF, and IL-1, the inhibitory action is likely directed at an early step in TNF receptor signaling. In view of the many similarities in the actions of TNF and IL-1 (30, 31), it is particularly interesting that NaSal affects differentially JNK activation induced by these two cytokines, suggesting that the inhibitory action occurs before the convergence of the TNF and IL-1 signaling pathways, probably at a TNF receptor-proximal site. Recent work has demonstrated the existence of 10 JNK isoforms (39). It will be of interest to determine which of these isoforms is activated by TNF and IL-1 in FS-4 fibroblasts, and whether the activation of these isoforms is equally susceptible to inhibition by NaSal. Our results do not support the view that the inhibition of TNF actions by NaSal can be ascribed to a nonspecific blocking of cellular kinases (40). The mechanism of p38 kinase activation by NaSal is unknown; NaSal may enhance the function of upstream p38 kinase activators [e.g., the MKK-3 or MKK-6 kinases (41, 42)], or it could inhibit phosphatase activity, thereby preventing p38 kinase deactivation. Whereas JNK and p38 MAPKs are often coordinately regulated by the same stimuli (4–9), our findings support the notion that these two MAPK subfamilies are part of separate signaling pathways.
NaSal treatment of FS-4 fibroblasts induced apoptosis, which was largely prevented by the selective p38 kinase inhibitor SB-203580, thus establishing an essential role for p38 kinase in NaSal-induced apoptosis. As ERKs were shown to protect cells from apoptosis in some systems (16, 43, 44), it is possible that the ability of NaSal to inhibit ERK activation (ref. 19; see Fig. 3A Top) contributes to cytotoxicity. Induction of apoptosis by NaSal may also be related to the demonstrated ability of NaSal to inhibit NF-κB activity (17, 18). Several recent reports demonstrated that inhibition of NF-κB promotes apoptosis induced by TNF and some other agents, whereas NF-κB activation protects cells from cell death (45–48). NaSal and some other NSAIDs (e.g., indomethacin and sulindac sulfate) were shown to induce apoptosis in colon cancer cells by a mechanism that may be unrelated to the ability of these drugs to inhibit prostaglandin synthesis (49–53). The widely documented chemopreventive and antineoplastic effects of NSAIDs in the colon (20, 21) have been linked to the induction of apoptosis by these drugs (22). Activation of p38 kinase and resulting apoptosis could be a common denominator in these antineoplastic actions of NSAIDs. It will be interesting to determine whether NaSal induces p38 activation and ensuing apoptosis in neoplastic cells and whether different NSAIDs share the ability to activate the p38 kinase. A related question is whether p38 kinase activation, along with the inhibition of TNF actions, plays a role in the anti-inflammatory actions of these drugs.
Acknowledgments
We thank John C. Lee, Peter Young, David Ron, Jiahuai Han, Arturo Zychlinsky, and Joseph Schlessinger for reagents and helpful discussions; Deborah Alpert for critical reading of the manuscript; Angel Feliciano for technical assistance; and Ilene Totillo for preparing the manuscript. This work was supported by National Institutes of Health Grants CA49731 (J.V.), CA42568 (C.B.), and DK49207 (E.Y.S.); the American Diabetes Association (E.Y.S.); and a predoctoral fellowship from National Institutes of Health Training Grant 5-T32-CA09161 (P.S.).
ABBREVIATIONS
- TNF
tumor necrosis factor
- MAPK
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- NaSal
sodium salicylate
- ERK
extracellular signal-regulated kinase
- EGF
epidermal growth factor
- IL-1
interleukin 1
- PDGF
platelet-derived growth factor
- GST
glutathione S-transferase
- NSAID
nonsteroidal anti-inflammatory drug
References
- 1.Cobb M H, Goldsmith E J. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 2.Cano E, Mahadevan L C. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 3.Marshall C J. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 4.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 5.Dérijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 6.Minden A, Lin A, Smeal T, Dérijard B, Cobb M, Davis R, Karin M. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han J, Lee J-D, Bibbs L, Ulevitch R J. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 8.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 9.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 10.Van Lint J, Agostinis P, Vandevoorde V, Haegeman G, Fiers W, Merlevede W, Vandenheede J R. J Biol Chem. 1992;267:25916–25921. [PubMed] [Google Scholar]
- 11.Vietor I, Schwenger P, Li W, Schlessinger J, Vilcek J. J Biol Chem. 1993;268:18994–18999. [PubMed] [Google Scholar]
- 12.Raines M A, Kolesnick R N, Golde D W. J Biol Chem. 1993;268:14572–14575. [PubMed] [Google Scholar]
- 13.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 14.Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee J C, Haegeman G, Cohen P, Fiers W. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 15.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 16.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 17.Kopp E, Ghosh S. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 18.Pierce J W, Read M A, Ding H, Luscinskas F W, Collins T. J Immunol. 1996;156:3961–3969. [PubMed] [Google Scholar]
- 19.Schwenger P, Skolnik E Y, Vilcek J. J Biol Chem. 1996;271:8089–8094. doi: 10.1074/jbc.271.14.8089. [DOI] [PubMed] [Google Scholar]
- 20.Giardiello F M, Hamilton S R, Krush A J, Piantadosi S, Hylind L M, Celano P, Booker S V, Robinson C R, Offerhaus G J. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 21.Giovannucci E, Egan K M, Hunter D J, Stampfer M J, Colditz G A, Willett W C, Speizer F E. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 22.Pasricha P J, Bedi A, O’Connor K, Rashid A, Akhtar A J, Zahurak M L, Piantadosi S, Hamilton S R, Giardiello F M. Gastroenterology. 1995;109:994–998. doi: 10.1016/0016-5085(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 23.Vilcek J, Palombella V J, Henriksen-DeStefano D, Swenson C, Feinman R, Hirai M, Tsujimoto M. J Exp Med. 1986;163:632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibi M, Lin A, Smeal T, Minden A, Karin M. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 25.Lin J-X, Vilcek J. J Biol Chem. 1987;262:11908–11911. [PubMed] [Google Scholar]
- 26.Sciavolino P J, Lee T H, Vilcek J. J Biol Chem. 1994;269:21627–21634. [PubMed] [Google Scholar]
- 27.Lee J C, Young P R. J Leukocyte Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 28.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 29.Prichett W, Hand A, Sheilds J, Dunnington D. J Inflam. 1995;45:97–105. [PubMed] [Google Scholar]
- 30.Le J, Vilcek J. Lab Invest. 1987;56:234–248. [PubMed] [Google Scholar]
- 31.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 32.Gille H, Sharrocks A D, Shaw P E. Nature (London) 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 33.Marais R, Wynne J, Treisman R. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 34.Zinck R, Cahill M A, Kracht M, Sachsenmaier C, Hipskind R A, Nordheim A. Mol Cell Biol. 1995;15:4930–4938. doi: 10.1128/mcb.15.9.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graves J D, Draves K E, Craxton A, Saklatvala J, Krebs E G, Clark E A. Proc Natl Acad Sci USA. 1996;93:13814–13818. doi: 10.1073/pnas.93.24.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Orsini M J, Lee J C, McDonnell P C, Debouck C, Young P R. J Biol Chem. 1996;271:30864–30869. doi: 10.1074/jbc.271.48.30864. [DOI] [PubMed] [Google Scholar]
- 37.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirstein M, Baglioni C. J Biol Chem. 1986;261:9565–9567. [PubMed] [Google Scholar]
- 39.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Dérijard B, Davis R J. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 40.Frantz B, O’Neill E A. Science. 1995;270:2017–2019. doi: 10.1126/science.270.5244.2017. [DOI] [PubMed] [Google Scholar]
- 41.Dérijard B, Raingeaud J, Barrett T, Wu I-H, Han J, Ulevitch R J, Davis R J. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 42.Raingeaud J, Whitmarsh A J, Barrett T, Dérijard B, Davis R J. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada T, Horiuchi M, Dzau V J. Proc Natl Acad Sci USA. 1996;93:156–160. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinoshita T, Yokota T, Arai K, Miyajima A. EMBO J. 1995;14:266–275. doi: 10.1002/j.1460-2075.1995.tb07000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beg A A, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 46.Liu Z-g, Hsu H, Goeddel D V, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 47.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 48.Wang C-Y, Mayo M W, Baldwin A S., Jr Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 49.Elder D J E, Hague A, Hicks D J, Paraskeva C. Cancer Res. 1996;56:2273–2276. [PubMed] [Google Scholar]
- 50.Shiff S J, Qiao L, Tsai L-L, Rigas B. J Clin Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piazza G A, Kulchak Rahm A L, Krutzsch M, Sperl G, Paranka N S, Gross P H, Brendel K, Burt R W, Alberts D S, Pamukcu R, Ahnen D J. Cancer Res. 1995;55:3110–3116. [PubMed] [Google Scholar]
- 52.Shiff S J, Koutsos M I, Qiao L, Rigas B. Exp Cell Res. 1996;222:179–188. doi: 10.1006/excr.1996.0023. [DOI] [PubMed] [Google Scholar]
- 53.Goldberg Y, Nassif I I, Pittas A, Tsai L-L, Dynlacht B D, Rigas B, Shiff S J. Oncogene. 1996;12:893–901. [PubMed] [Google Scholar]