Abstract
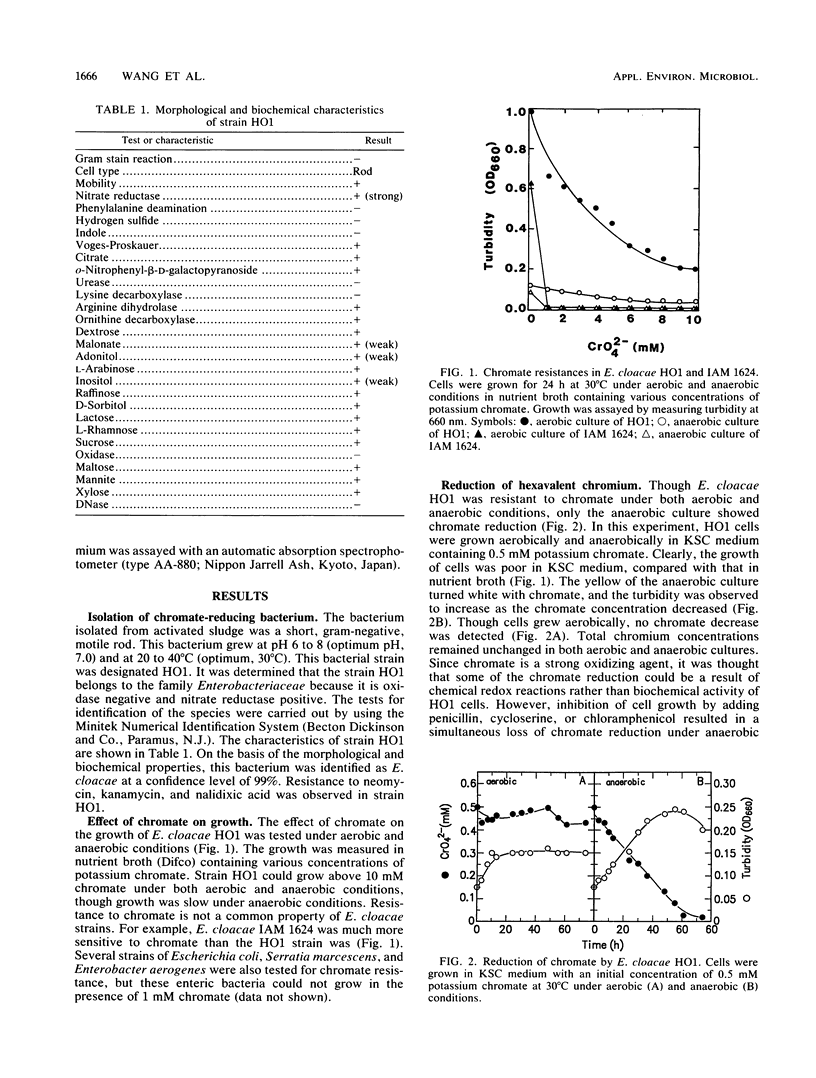
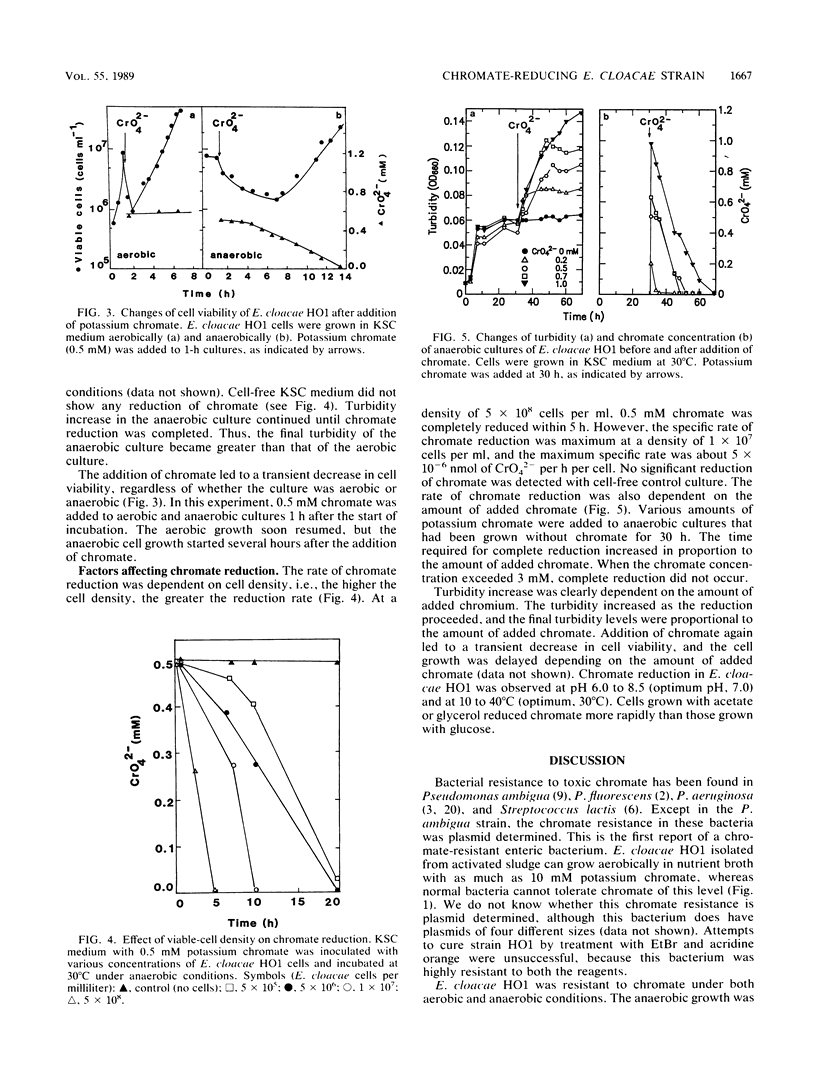
An Enterobacter cloacae strain (HO1) capable of reducing hexavalent chromium (chromate) was isolated from activated sludge. This bacterium was resistant to chromate under both aerobic and anaerobic conditions. Only the anaerobic culture of the E. cloacae isolate showed chromate reduction. In the anaerobic culture, yellow turned white with chromate and the turbidity increased as the reduction proceeded, suggesting that insoluble chromium hydroxide was formed. E. cloacae is likely to utilize toxic chromate as an electron acceptor anaerobically because (i) the anaerobic growth of E. cloacae HO1 accompanied the decrease of toxic chromate in culture medium, (ii) the chromate-reducing activity was rapidly inhibited by oxygen, and (iii) the reduction occurred more rapidly in glycerol- or acetate-grown cells than in glucose-grown cells. The chromate reduction in E. cloacae HO1 was observed at pH 6.0 to 8.5 (optimum pH, 7.0) and at 10 to 40°C (optimum, 30°C).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bopp L. H., Chakrabarty A. M., Ehrlich H. L. Chromate resistance plasmid in Pseudomonas fluorescens. J Bacteriol. 1983 Sep;155(3):1105–1109. doi: 10.1128/jb.155.3.1105-1109.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Gustafson J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl Environ Microbiol. 1976 Oct;32(4):567–571. doi: 10.1128/aem.32.4.567-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong M., González E., Barra R., Vásquez C. Purification and biochemical characterization of tellurite-reducing activities from Thermus thermophilus HB8. J Bacteriol. 1988 Jul;170(7):3269–3273. doi: 10.1128/jb.170.7.3269-3273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard T. L., Telford J. N., Williams H. H. Detection of selenium deposits in Escherichia coli by electron microscopy. J Bacteriol. 1974 Sep;119(3):1057–1060. doi: 10.1128/jb.119.3.1057-1060.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva E. V., Lialikova N. N. Vosstanovlenie krokoita kul'turoi Pseudomonas chromatophila sp. nov. Mikrobiologiia. 1979 May-Jun;48(3):517–522. [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988 Jun 3;240(4857):1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J., FALCONE G. ENZYMATIC REDUCTION OF SELENITE. J Bacteriol. 1963 Apr;85:763–771. doi: 10.1128/jb.85.4.763-771.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake H., Cervantes C., Silver S. Decreased chromate uptake in Pseudomonas fluorescens carrying a chromate resistance plasmid. J Bacteriol. 1987 Aug;169(8):3853–3856. doi: 10.1128/jb.169.8.3853-3856.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson B. H., Barkay T., Colwell R. R. Role of plasmids in mercury transformation by bacteria isolated from the aquatic environment. Appl Environ Microbiol. 1979 Sep;38(3):478–485. doi: 10.1128/aem.38.3.478-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C., Von Klopotek A. Enzymatic reduction of iron oxide by fungi. Appl Microbiol. 1969 Jul;18(1):41–43. doi: 10.1128/am.18.1.41-43.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli F. L., De Flora S. Toxicity and mutagenicity of hexavalent chromium on Salmonella typhimurium. Appl Environ Microbiol. 1977 Apr;33(4):805–809. doi: 10.1128/aem.33.4.805-809.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to boron and chromium compounds in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Apr;13(4):637–640. doi: 10.1128/aac.13.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- TERAI T., KAMAHORA T., YAMAMURA Y. Tellurite reductase from Mycobacterium avium. J Bacteriol. 1958 May;75(5):535–539. doi: 10.1128/jb.75.5.535-539.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUCKER F. L., WALPER J. F., APPLEMAN M. D., DONOHUE J. Complete reduction of tellurite to pure tellurium metal by microorganisms. J Bacteriol. 1962 Jun;83:1313–1314. doi: 10.1128/jb.83.6.1313-1314.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Ehrlich H. L. Bacteriology of manganese nodules: III. Reduction of MnO(2) by two strains of nodule bacteria. Appl Microbiol. 1968 May;16(5):695–702. doi: 10.1128/am.16.5.695-702.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venitt S., Levy L. S. Mutagenicity of chromates in bacteria and its relevance to chromate carcinogenesis. Nature. 1974 Aug 9;250(5466):493–495. doi: 10.1038/250493a0. [DOI] [PubMed] [Google Scholar]


