Abstract
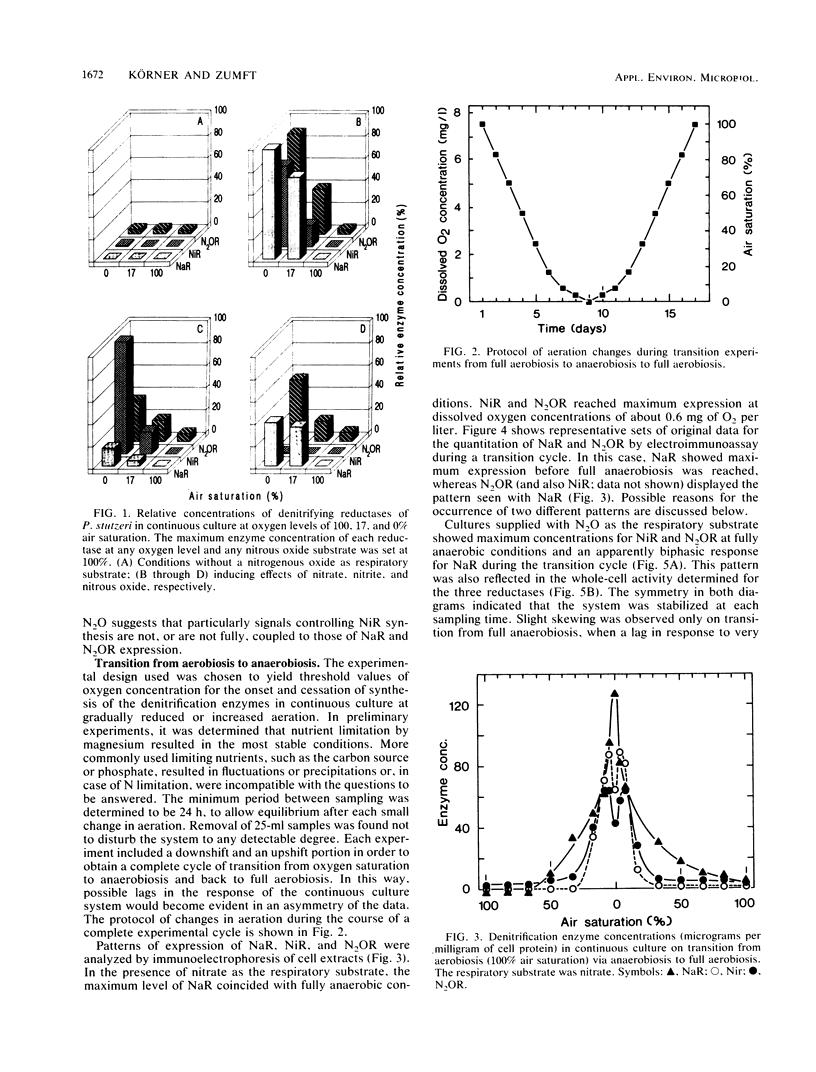
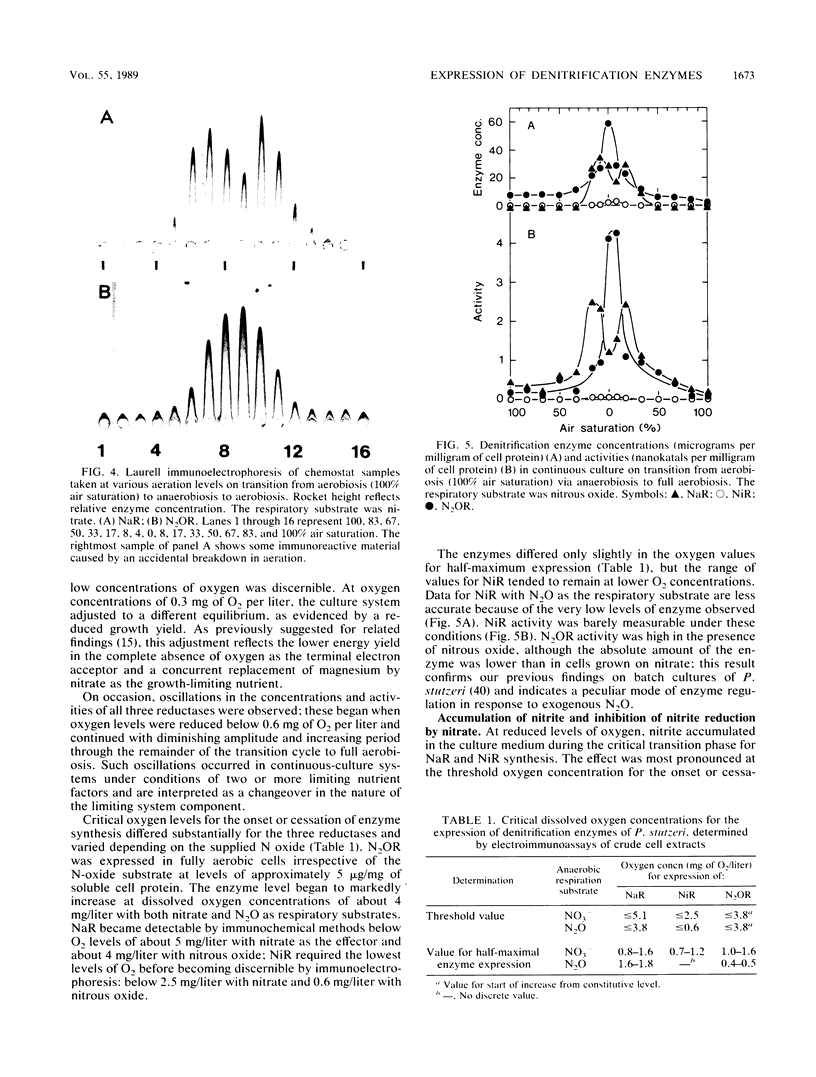
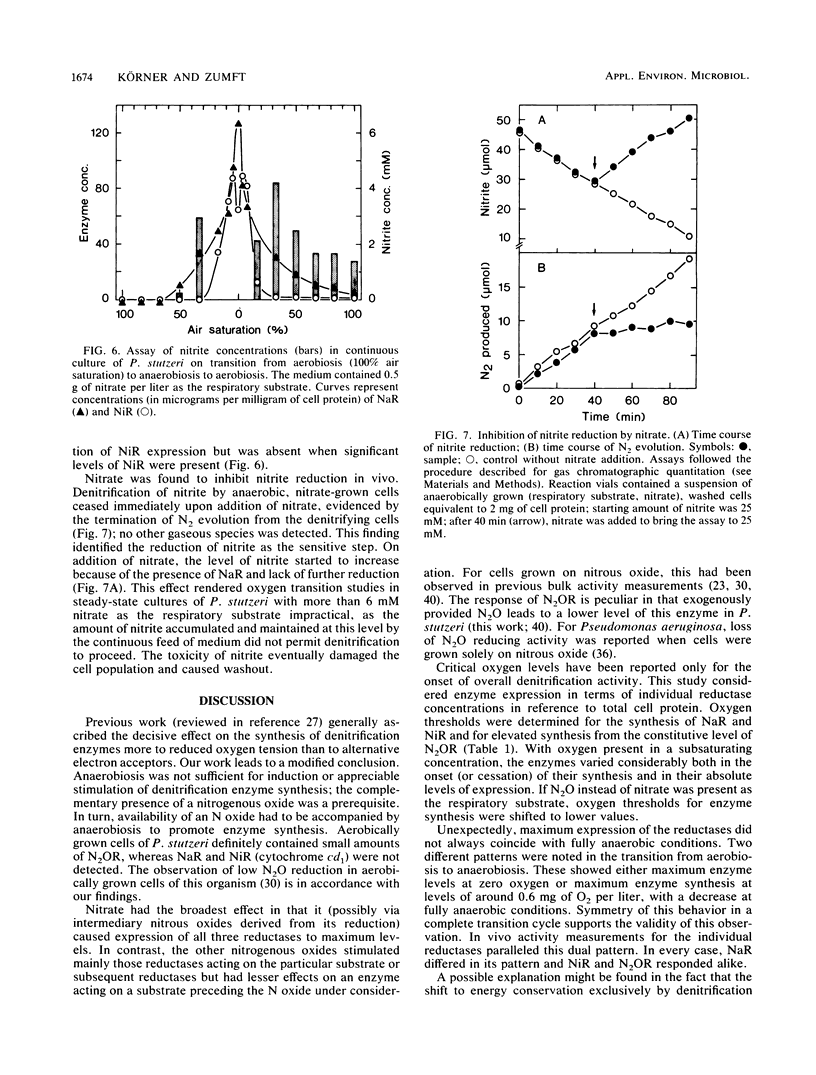
The onset and cessation of the synthesis of denitrification enzymes of Pseudomonas stutzeri were investigated by using continuous culture and defined dissolved oxygen levels covering the full range of transition from air saturation to complete anaerobiosis. Expression of nitrate reductase, nitrite reductase (cytochrome cd1), and N2O reductase was controlled by discrete oxygen levels and by the nature of the nitrogenous oxide available for respiration. N2O reductase was synthesized constitutively at a low level; for enhanced expression, oxygen concentrations were required to decrease below 5 mg of O2 per liter. The threshold values for synthesis of nitrate reductase and cytochrome cd1 in the presence of nitrate were ca. 5 and ca. 2.5 mg of O2 per liter, respectively. With nitrous oxide as the respiratory substrate, nitrite reductase was again the most sensitive to oxygen concentration; however, thresholds for all denitrification enzymes shifted to lower oxygen levels. Whereas the presence of nitrate resulted in maximum expression and nearly uniform induction of all reductases, nitrite and nitrous oxide stimulated preferably the respective enzyme catalyzing reduction. In the absence of a nitrogenous oxide, anaerobiosis did not induce enzyme synthesis to any significant degree. The accumulation of nitrite seen during both the aerobic-anaerobic and anaerobic-aerobic transition phases was caused by the differences in onset or cessation of synthesis of nitrate and nitrite reductases and an inhibitory effect of nitrate on nitrite reduction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazylinski D. A., Blakemore R. P. Denitrification and Assimilatory Nitrate Reduction in Aquaspirillum magnetotacticum. Appl Environ Microbiol. 1983 Nov;46(5):1118–1124. doi: 10.1128/aem.46.5.1118-1124.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder K., Burke K. A., Lascelles J. Induction of nitrate reductase and membrane cytochromes in wild type and chlorate-resistant Paracoccus denitrificans. Arch Microbiol. 1980 Jun;126(2):149–153. doi: 10.1007/BF00511220. [DOI] [PubMed] [Google Scholar]
- Coyle C. L., Zumft W. G., Kroneck P. M., Körner H., Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina. Purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985 Dec 16;153(3):459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I., Betlach M., Kritikos G. The effect of oxygen on denitrification during steady-state growth of Paracoccus halodenitrificans. Arch Microbiol. 1984 Jan;137(1):74–78. doi: 10.1007/BF00425811. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I., Tomlinson G. A. The enzymes associated with denitrification. Annu Rev Microbiol. 1988;42:231–261. doi: 10.1146/annurev.mi.42.100188.001311. [DOI] [PubMed] [Google Scholar]
- John P. Aerobic and anaerobic bacterial respiration monitored by electrodes. J Gen Microbiol. 1977 Jan;98(1):231–238. doi: 10.1099/00221287-98-1-231. [DOI] [PubMed] [Google Scholar]
- Koike I., Hattori A. Growth yield of a denitrifying bacterium, Pseudomonas denitrificans, under aerobic and denitrifying conditions. J Gen Microbiol. 1975 May;88(1):1–10. doi: 10.1099/00221287-88-1-1. [DOI] [PubMed] [Google Scholar]
- Körner H., Frunzke K., Döhler K., Zumft W. G. Immunochemical patterns of distribution of nitrous oxide reductase and nitrite reductase (cytochrome cd1) among denitrifying pseudomonads. Arch Microbiol. 1987 Jun;148(1):20–24. doi: 10.1007/BF00429641. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Frunzke K., Zumft W. G. Modulation by copper of the products of nitrite respiration in Pseudomonas perfectomarinus. J Bacteriol. 1982 Mar;149(3):816–823. doi: 10.1128/jb.149.3.816-823.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T. Studies on denitrification. 8. Some properties of the N2O-anaerobically grown cell. J Biochem. 1971 Jun;69(6):991–1001. doi: 10.1093/oxfordjournals.jbchem.a129572. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Riley P. S., Cox C. D., Jr Separate nitrite, nitric oxide, and nitrous oxide reducing fractions from Pseudomonas perfectomarinus. J Bacteriol. 1971 May;106(2):356–361. doi: 10.1128/jb.106.2.356-361.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Riley P. S. Suppression by nitrate of enzymatic reduction of nitric oxide. Proc Soc Exp Biol Med. 1969 Oct;132(1):258–260. doi: 10.3181/00379727-132-34192. [DOI] [PubMed] [Google Scholar]
- Robertson L. A., Kuenen J. G. Aerobic denitrification--old wine in new bottles? Antonie Van Leeuwenhoek. 1984;50(5-6):525–544. doi: 10.1007/BF02386224. [DOI] [PubMed] [Google Scholar]
- Robertson L. A., van Niel E. W., Torremans R. A., Kuenen J. G. Simultaneous Nitrification and Denitrification in Aerobic Chemostat Cultures of Thiosphaera pantotropha. Appl Environ Microbiol. 1988 Nov;54(11):2812–2818. doi: 10.1128/aem.54.11.2812-2818.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. W., Bazylinski D. A., Hollocher T. C. Loss of N2O reductase activity as an explanation for poor growth of Pseudomonas aeruginosa on N2O. Appl Environ Microbiol. 1987 Sep;53(9):2045–2049. doi: 10.1128/aem.53.9.2045-2049.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Döhler K., Körner H. Isolation and characterization of transposon Tn5-induced mutants of Pseudomonas perfectomarina defective in nitrous oxide respiration. J Bacteriol. 1985 Sep;163(3):918–924. doi: 10.1128/jb.163.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Döhler K., Körner H., Löchelt S., Viebrock A., Frunzke K. Defects in cytochrome cd1-dependent nitrite respiration of transposon Tn5-induced mutants from Pseudomonas stutzeri. Arch Microbiol. 1988;149(6):492–498. doi: 10.1007/BF00446750. [DOI] [PubMed] [Google Scholar]
- van Verseveld H. W., Meijer E. M., Stouthamer A. H. Energy conservation during nitrate respiration in Paracoccus denitrificans. Arch Microbiol. 1977 Feb 4;112(1):17–23. doi: 10.1007/BF00446649. [DOI] [PubMed] [Google Scholar]