Abstract
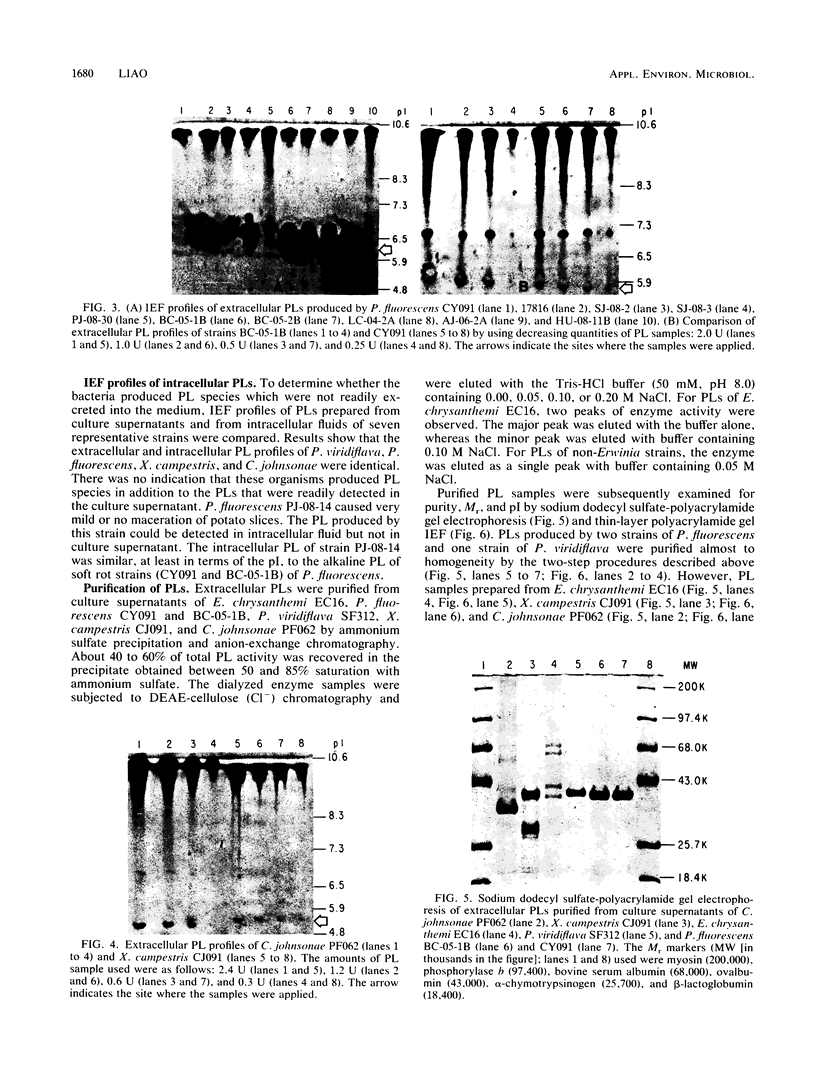
Isoelectric focusing (IEF) profiles of pectate lyases (PLs) produced by five different groups of soft rot bacteria were analyzed by using the combined techniques of thin-layer polyacrylamide gel IEF and agarose-pectate overlay activity staining. Four strains of soft rot Erwinia spp. produced three or more PL isozymes. All of eight Pseudomonas viridiflava strains examined produced one single PL with a pI of 9.7. All 10 of Pseudomonas fluorescens strains produced two PLs; the major one had a pI of 10.0 and the minor one had a pI of 6.7. A single PL with a pI of greater than or equal to 10.0 was detected in one strain each of Xanthomonas campestris and Cytophaga johnsonae. PLs of six representative strains were purified from culture supernatants by ammonium sulfate precipitation and anion-exchange chromatography. All purified PL samples macerated potato slices, but to different degrees. The Mrs of alkaline PLs produced by P. viridiflava, P. fluorescens, X. campestris, and C. johnsonae were estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to be 42,000, 41,000, 41,500, and 35,000, respectively. IEF profiles of PLs were distinct among the bacterial species. Profiles of non-Erwinia spoilage bacteria were considerably simpler than those of Erwinia spp. The PL with an alkaline pI appeared to be the principal or the sole enzymatic factor involved in tissue maceration caused by most strains of soft rot bacteria.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barras F., Thurn K. K., Chatterjee A. K. Resolution of four pectate lyase structural genes of Erwinia chrysanthemi (EC16) and characterization of the enzymes produced in Escherichia coli. Mol Gen Genet. 1987 Sep;209(2):319–325. doi: 10.1007/BF00329660. [DOI] [PubMed] [Google Scholar]
- Bertheau Y., Madgidi-Hervan E., Kotoujansky A., Nguyen-The C., Andro T., Coleno A. Detection of depolymerase isoenzymes after electrophoresis or electrofocusing, or in titration curves. Anal Biochem. 1984 Jun;139(2):383–389. doi: 10.1016/0003-2697(84)90022-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Buchanan G. E., Behrens M. K., Starr M. P. Synthesis and excretion of polygalacturonic acid trans-eliminase in Erwinia, Yersinia, and Klebsiella species. Can J Microbiol. 1979 Jan;25(1):94–102. doi: 10.1139/m79-014. [DOI] [PubMed] [Google Scholar]
- Collmer A., Schoedel C., Roeder D. L., Ried J. L., Rissler J. F. Molecular cloning in Escherichia coli of Erwinia chrysanthemi genes encoding multiple forms of pectate lyase. J Bacteriol. 1985 Mar;161(3):913–920. doi: 10.1128/jb.161.3.913-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Lyon G. D., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXV. Endopolygalacturonic Acid Lyase from Erwinia carotovora Elicits Phytoalexin Accumulation by Releasing Plant Cell Wall Fragments. Plant Physiol. 1984 Jan;74(1):52–60. doi: 10.1104/pp.74.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A. The trans-eliminative breakdown of Na-polygalacturonate by Pseudomonas fluorescens. Antonie Van Leeuwenhoek. 1965;31(3):323–340. doi: 10.1007/BF02045912. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S. Structure of two pectate lyase genes from Erwinia chrysanthemi EC16 and their high-level expression in Escherichia coli. J Bacteriol. 1986 Nov;168(2):595–606. doi: 10.1128/jb.168.2.595-606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liao C. H., Wells J. M. Properties of Cytophaga johnsonae strains causing spoilage of fresh produce at food markets. Appl Environ Microbiol. 1986 Dec;52(6):1261–1265. doi: 10.1128/aem.52.6.1261-1265.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manulis S., Kobayashi D. Y., Keen N. T. Molecular cloning and sequencing of a pectate lyase gene from Yersinia pseudotuberculosis. J Bacteriol. 1988 Apr;170(4):1825–1830. doi: 10.1128/jb.170.4.1825-1830.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Pectic enzymes of pseudomonas marginalis. Phytopathology. 1966 Dec;56(12):1414–1415. [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonic acid trans-eliminase of Xanthomonas campestris. Biochem J. 1967 Jul;104(1):178–185. doi: 10.1042/bj1040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. H., Schoedel C., Keen N. T., Collmer A. Multiplication and Virulence in Plant Tissues of Escherichia coli Clones Producing Pectate Lyase Isozymes PLb and PLe at High Levels and of an Erwinia chrysanthemi Mutant Deficient in PLe. Appl Environ Microbiol. 1987 Oct;53(10):2315–2320. doi: 10.1128/aem.53.10.2315-2320.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. Activity stain for rapid characterization of pectic enzymes in isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gels. Appl Environ Microbiol. 1985 Sep;50(3):615–622. doi: 10.1128/aem.50.3.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. Comparison of pectic enzymes produced by Erwinia chrysanthemi, Erwinia carotovora subsp. carotovora, and Erwinia carotovora subsp. atroseptica. Appl Environ Microbiol. 1986 Aug;52(2):305–310. doi: 10.1128/aem.52.2.305-310.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder D. L., Collmer A. Marker-exchange mutagenesis of a pectate lyase isozyme gene in Erwinia chrysanthemi. J Bacteriol. 1985 Oct;164(1):51–56. doi: 10.1128/jb.164.1.51-56.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlemmer A. F., Ware C. F., Keen N. T. Purification and characterization of a pectin lyase produced by Pseudomonas fluorescens W51. J Bacteriol. 1987 Oct;169(10):4493–4498. doi: 10.1128/jb.169.10.4493-4498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward O. P., Fogarty W. M. Polygalacturonate lyase production by Bacillus subtilis and Flavobacterium pectinovorum. Appl Microbiol. 1974 Feb;27(2):346–350. doi: 10.1128/am.27.2.346-350.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M., Hankin L. Regulation of pectate lyase synthesis in Pseudomonas fluorescens and Erwinia carotovora. J Bacteriol. 1970 Oct;104(1):13–18. doi: 10.1128/jb.104.1.13-18.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]