Abstract
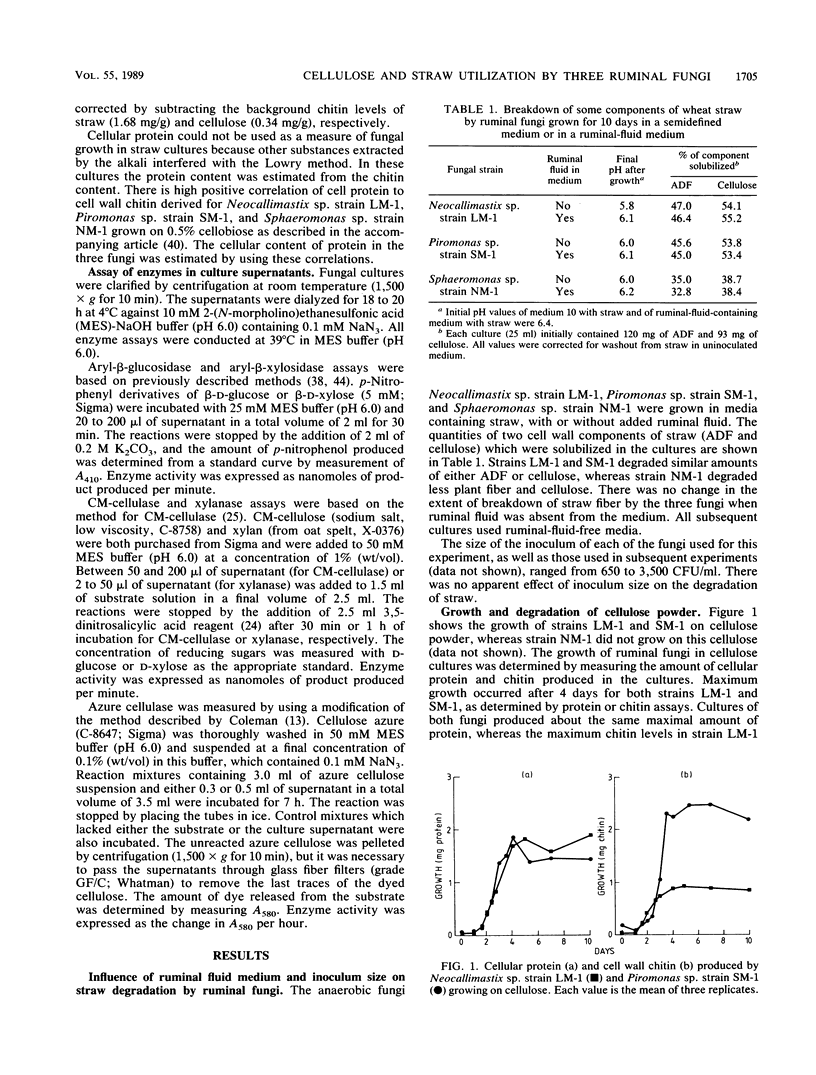
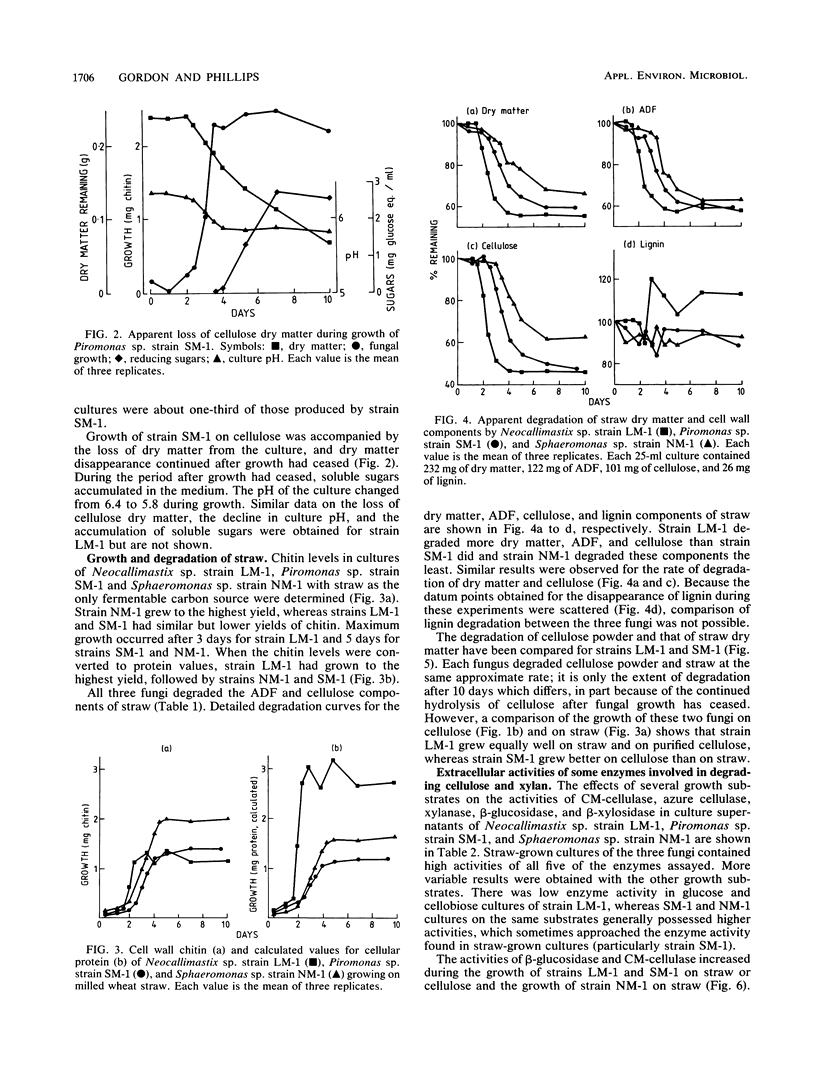
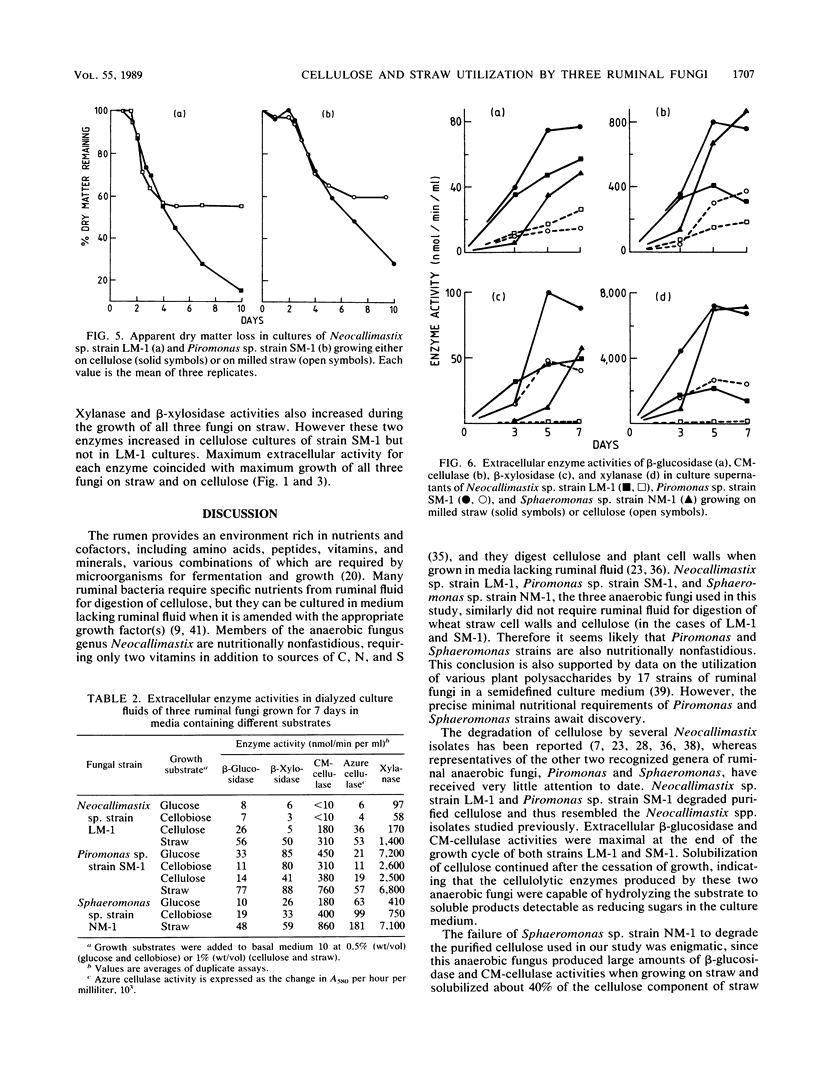
Three different ruminal fungi, a Neocallimastix sp. (strain LM-1), a Piromonas sp. (strain SM-1), and a Sphaeromonas sp. (strain NM-1), were grown anaerobically in liquid media which contained a suspension of either 1% (wt/vol) purified cellulose or finely milled wheat straw as the source of fermentable carbon. Fungal biomass was estimated by using cell wall chitin or cellular protein in cellulose cultures and chitin in straw cultures. Both strains LM-1 and SM-1 degraded cellulose with a concomitant increase in fungal biomass. Maximum growth of both fungi occurred after incubation for 4 days, and the final yield of protein was the same for both fungi. Cellulose degradation continued after growth ceased. Strain NM-1 failed to grow in the cellulose medium. All three anaerobic fungi grew in the straw-containing medium, and loss of dry weight from the cultures indicated degradation of straw to various degrees (LM-1 greater than SM-1 greater than NM-1). The total fiber component and the cellulose component of the straw were degraded in similar proportions, but the lignin component remained undegraded by any of the fungi. Maximum growth yield on straw occurred after 4 days for strain LM-1 and after 5 days for strains SM-1 and NM-1. The calculated yield of cellular protein for strain LM-1 was twice that of both strains SM-1 and NM-1. The cellular protein yield of strain SM-1 was the same in both cellulose and straw cultures. In contrast to cellulose, straw degradation ceased after the end of the growth phase.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Benner R. Degradation of polysaccharides and lignin by ruminal bacteria and fungi. Appl Environ Microbiol. 1988 May;54(5):1117–1125. doi: 10.1128/aem.54.5.1117-1125.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Borneman W. S., Windham W. R. Rumen fungi: morphological types from Georgia cattle and the attack on forage cell walls. Biosystems. 1988;21(3-4):385–391. doi: 10.1016/0303-2647(88)90037-8. [DOI] [PubMed] [Google Scholar]
- Akin D. E., Gordon G. L., Hogan J. P. Rumen bacterial and fungal degradation of Digitaria pentzii grown with or without sulfur. Appl Environ Microbiol. 1983 Sep;46(3):738–748. doi: 10.1128/aem.46.3.738-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Rumen anaerobic fungi of cattle and sheep. Appl Environ Microbiol. 1979 Jul;38(1):148–158. doi: 10.1128/aem.38.1.148-158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. C., Johnson B. R. Improved colorimetric determination of cell wall chitin in wood decay fungi. Appl Environ Microbiol. 1983 Jul;46(1):13–16. doi: 10.1128/aem.46.1.13-16.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joblin K. N. Isolation, enumeration, and maintenance of rumen anaerobic fungi in roll tubes. Appl Environ Microbiol. 1981 Dec;42(6):1119–1122. doi: 10.1128/aem.42.6.1119-1122.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl Environ Microbiol. 1987 Jun;53(6):1216–1223. doi: 10.1128/aem.53.6.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1985 May;49(5):1314–1322. doi: 10.1128/aem.49.5.1314-1322.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpin C. G. Invasion of plant tissue in the rumen by the flagellate Neocallimastix frontalis. J Gen Microbiol. 1977 Feb;98(2):423–430. doi: 10.1099/00221287-98-2-423. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol. 1975 Dec;91(2):249–262. doi: 10.1099/00221287-91-2-249. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Sphaeromonas communis. J Gen Microbiol. 1976 Jun;94(2):270–280. doi: 10.1099/00221287-94-2-270. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. The occurrence of chitin in the cell walls of the rumen organisms Neocallimastix frontalis, Piromonas communis and Sphaeromonas communis. J Gen Microbiol. 1977 Mar;99(1):215–218. doi: 10.1099/00221287-99-1-215. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. The rumen flagellate Piromonas communis: its life-history and invasion of plant material in the rumen. J Gen Microbiol. 1977 Mar;99(1):107–117. doi: 10.1099/00221287-99-1-107. [DOI] [PubMed] [Google Scholar]
- Pearce P. D., Bauchop T. Glycosidases of the rumen anaerobic fungus Neocallimastix frontalis grown on cellulosic substrates. Appl Environ Microbiol. 1985 May;49(5):1265–1269. doi: 10.1128/aem.49.5.1265-1269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. W., Gordon G. L. Growth characteristics on cellobiose of three different anaerobic fungi isolated from the ovine rumen. Appl Environ Microbiol. 1989 Jul;55(7):1695–1702. doi: 10.1128/aem.55.7.1695-1702.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. W., Gordon G. L. Sugar and polysaccharide fermentation by rumen anaerobic fungi from Australia, Britain and New Zealand. Biosystems. 1988;21(3-4):377–383. doi: 10.1016/0303-2647(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Stack R. J., Cotta M. A. Effect of 3-phenylpropanoic Acid on growth of and cellulose utilization by cellulolytic ruminal bacteria. Appl Environ Microbiol. 1986 Jul;52(1):209–210. doi: 10.1128/aem.52.1.209-210.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Glycoside hydrolase enzymes present in the zoospore and vegetative growth stages of the rumen fungi Neocallimastix patriciarum, Piromonas communis, and an unidentified isolate, grown on a range of carbohydrates. Can J Microbiol. 1987 May;33(5):427–434. doi: 10.1139/m87-072. [DOI] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Polysaccharide-degrading enzymes formed by three species of anaerobic rumen fungi grown on a range of carbohydrate substrates. Can J Microbiol. 1987 May;33(5):418–426. doi: 10.1139/m87-071. [DOI] [PubMed] [Google Scholar]


