Abstract
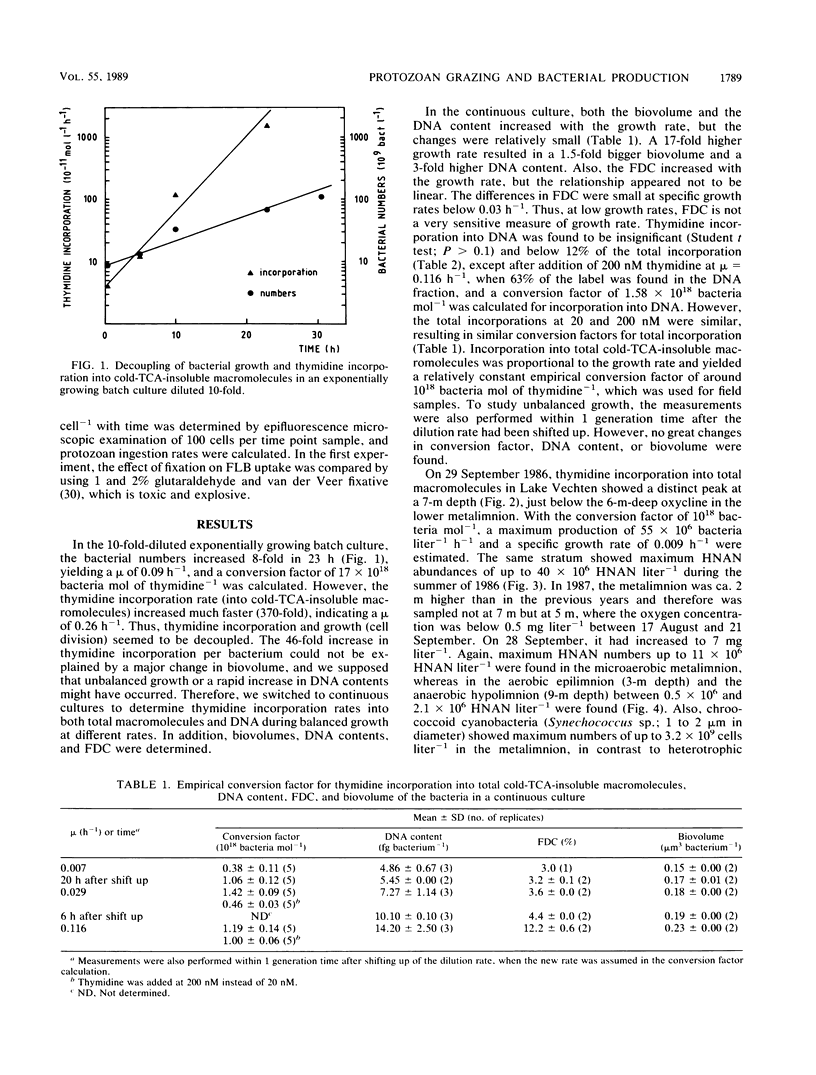
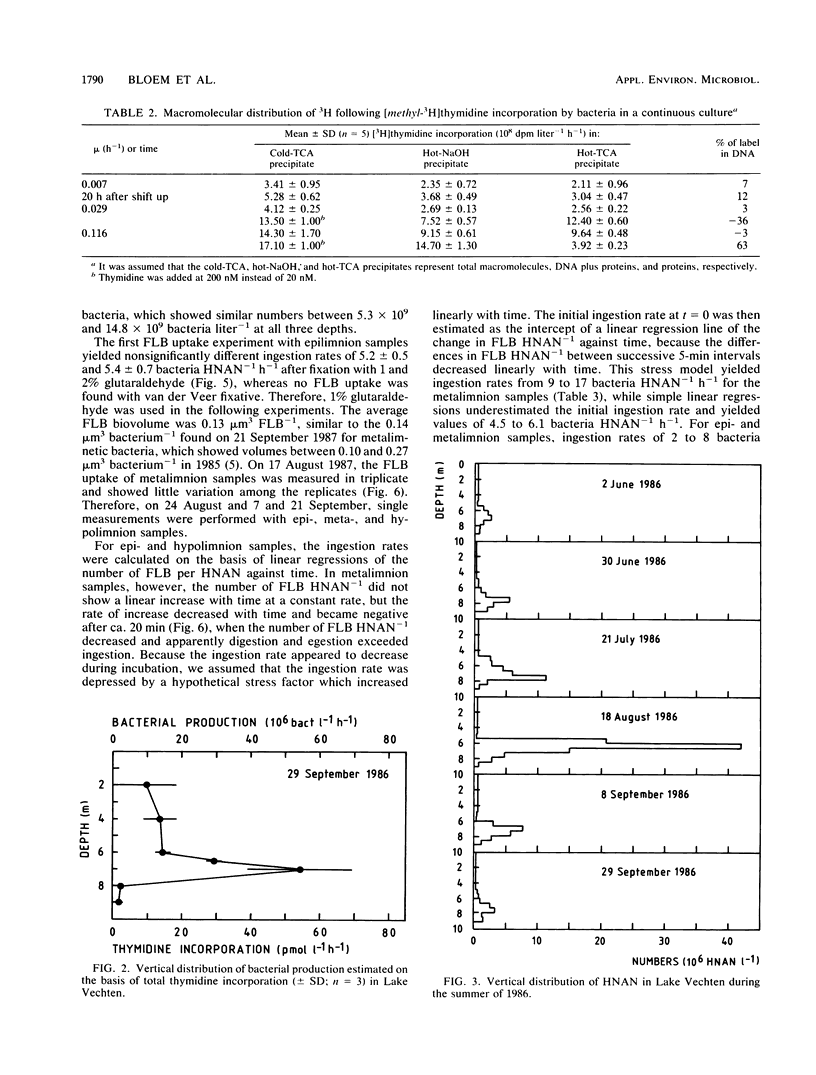
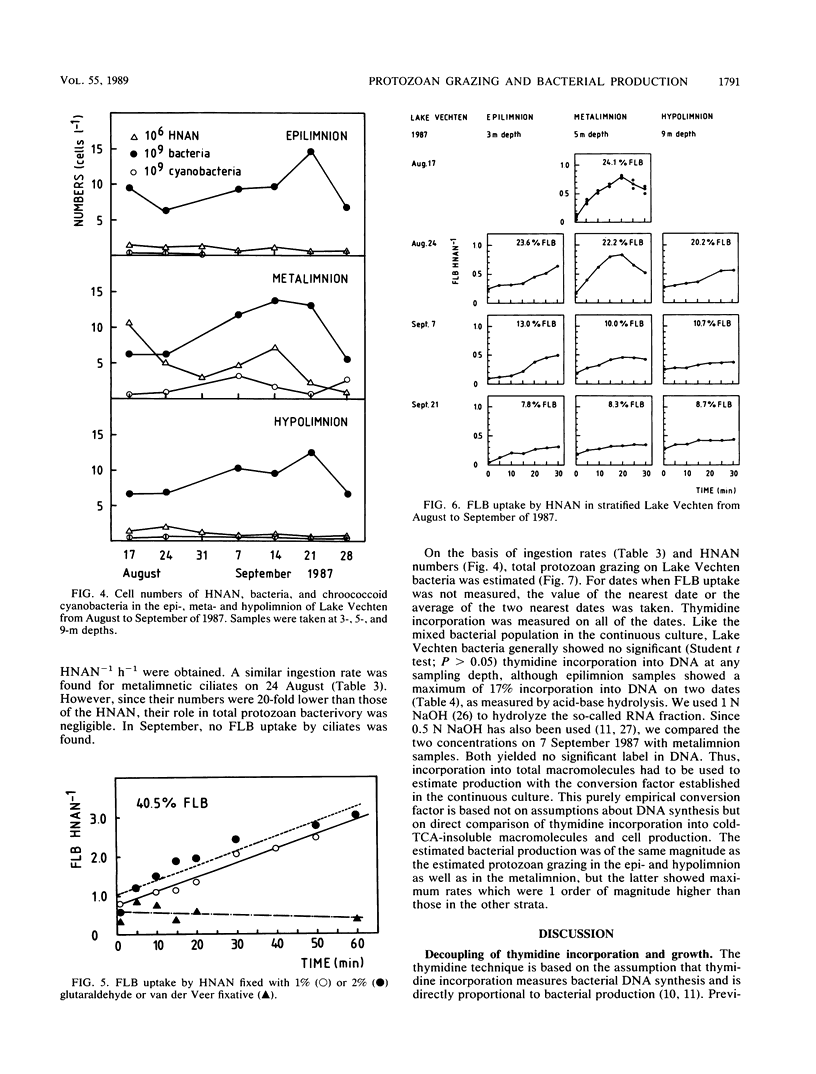
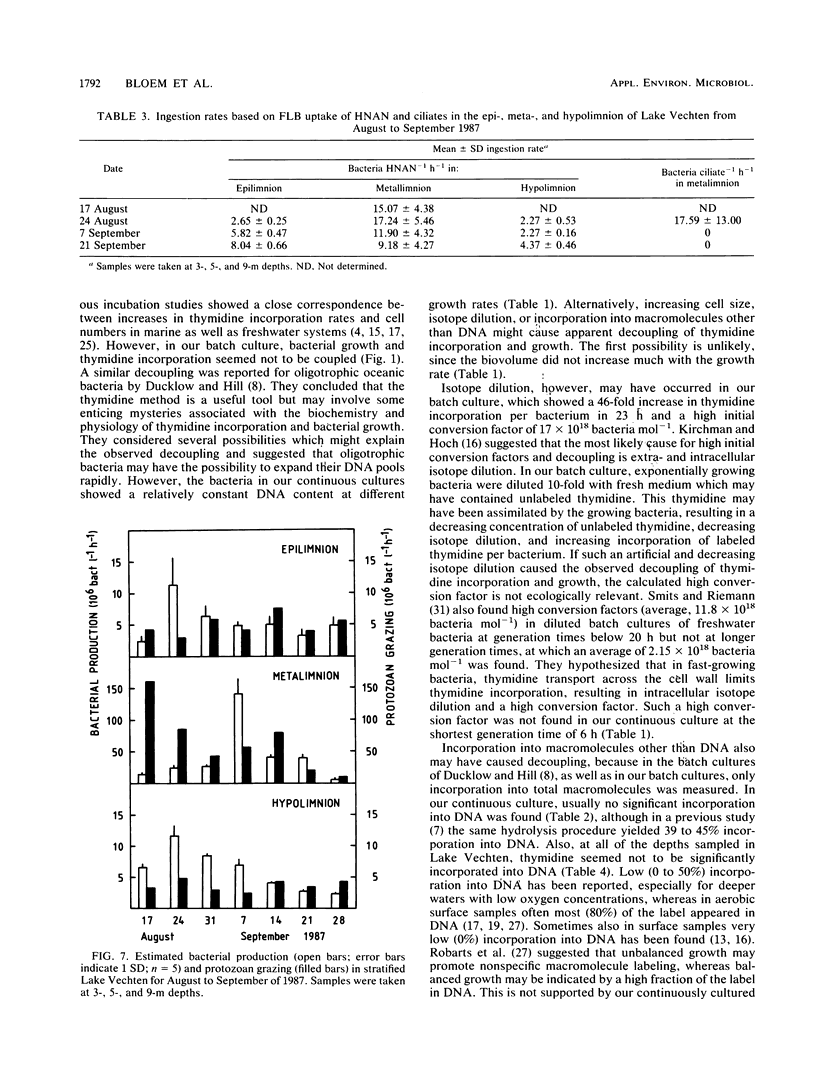
In stratified Lake Vechten, The Netherlands, protozoan grazing was estimated on the basis of uptake of fluorescently labeled bacteria and compared with bacterial production estimated on the basis of thymidine incorporation. By using a grazer-free mixed bacterial population from the lake in continuous culture, an empirical relationship between cell production and thymidine incorporation was established. Thymidine incorporation into total cold-trichloroacetic-acid-insoluble macromolecules yielded a relatively constant empirical conversion factor of ca. 1018 (range, 0.38 × 1018 to 1.42 × 1018) bacteria mol of thymidine−1 at specific growth rates (μ) ranging from 0.007 to 0.116 h−1. Although thymidine incorporation has been assumed to measure DNA synthesis thymidine incorporation appeared to underestimate the independently measured bacterial DNA synthesis by at least 1.5- to 13-fold, even if all incorporated label was assumed to be in DNA. However, incorporation into DNA was found to be insignificant as measured by conventional acid-base hydrolysis. Methodological problems of the thymidine technique are discussed. Like the cultures, Lake Vechten bacteria showed considerable thymidine incorporation into total macromolecules, but no significant incorporation into DNA was found by acid-base hydrolysis. This applied not only to the low-oxygen hypo- and metalimnion but also to the aerobic epilimnion. Thus, the established empirical conversion factor for thymidine incorporation into total macromolecules was used to estimate bacterial production. Maximum production rates (141 × 106 bacteria liter−1 h−1; μ, 0.012 h−1) were found in the metalimnion and were 1 order of magnitude higher than in the epi- and hypolimnion. In all three strata, the estimated bacterial production was roughly balanced by the estimated protozoan grazing. Heterotrophic nanoflagellates were the major consumers of the bacterial production and showed maximum numbers (up to 40 × 106 heterotrophic nanoflagellates liter−1) in the microaerobic metalimnion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem J., Bär-Gilissen M. J., Cappenberg T. E. Fixation, counting, and manipulation of heterotrophic nanoflagellates. Appl Environ Microbiol. 1986 Dec;52(6):1266–1272. doi: 10.1128/aem.52.6.1266-1272.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem J., Starink M., Bär-Gilissen M. J., Cappenberg T. E. Protozoan grazing, bacterial activity, and mineralization in two-stage continuous cultures. Appl Environ Microbiol. 1988 Dec;54(12):3113–3121. doi: 10.1128/aem.54.12.3113-3121.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström A., Larsson U., Hörstedt P., Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979 May;37(5):805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey W. H., Paul J. H. Underestimation of DNA synthesis by [h]thymidine incorporation in marine bacteria. Appl Environ Microbiol. 1988 Dec;54(12):3165–3168. doi: 10.1128/aem.54.12.3165-3168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Seasonal bacterial production in a dimictic lake as measured by increases in cell numbers and thymidine incorporation. Appl Environ Microbiol. 1985 Mar;49(3):492–500. doi: 10.1128/aem.49.3.492-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough R. J., Sanders R. W., Porter K. G., Kirchman D. L. Depth distribution of bacterial production in a stratified lake with an anoxic hypolimnion. Appl Environ Microbiol. 1986 Nov;52(5):992–1000. doi: 10.1128/aem.52.5.992-1000.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Myers B. Fluorometric determination of DNA in aquatic microorganisms by use of hoechst 33258. Appl Environ Microbiol. 1982 Jun;43(6):1393–1399. doi: 10.1128/aem.43.6.1393-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann B., Søndergaard M. Measurements of diel rates of bacterial secondary production in aquatic environments. Appl Environ Microbiol. 1984 Apr;47(4):632–638. doi: 10.1128/aem.47.4.632-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarts R. D., Wicks R. J., Sephton L. M. Spatial and Temporal Variations in Bacterial Macromolecule Labeling with [methyl-H]Thymidine in a Hypertrophic Lake. Appl Environ Microbiol. 1986 Dec;52(6):1368–1373. doi: 10.1128/aem.52.6.1368-1373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais P., Martinez J., Billen G., Vives-Rego J. Determining [H]Thymidine Incorporation into Bacterioplankton DNA: Improvement of the Method by DNase Treatment. Appl Environ Microbiol. 1987 Aug;53(8):1977–1979. doi: 10.1128/aem.53.8.1977-1979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits J. D., Riemann B. Calculation of cell production from [h]thymidine incorporation with freshwater bacteria. Appl Environ Microbiol. 1988 Sep;54(9):2213–2219. doi: 10.1128/aem.54.9.2213-2219.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


