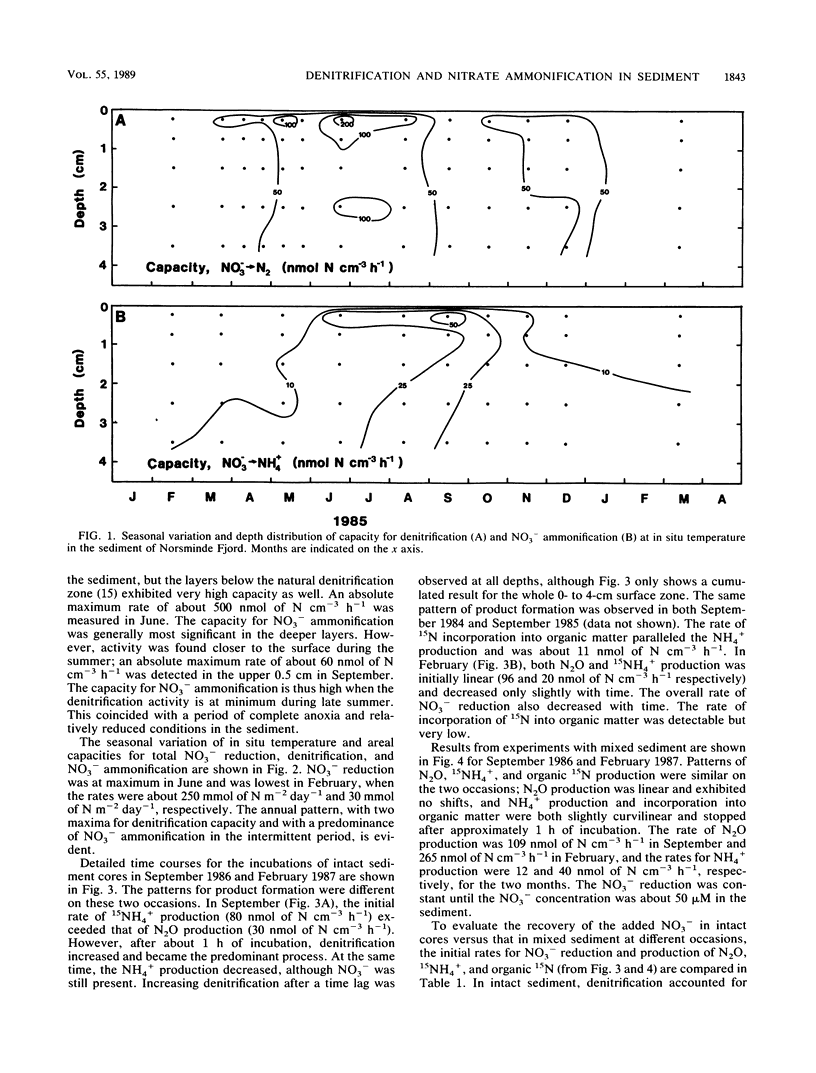
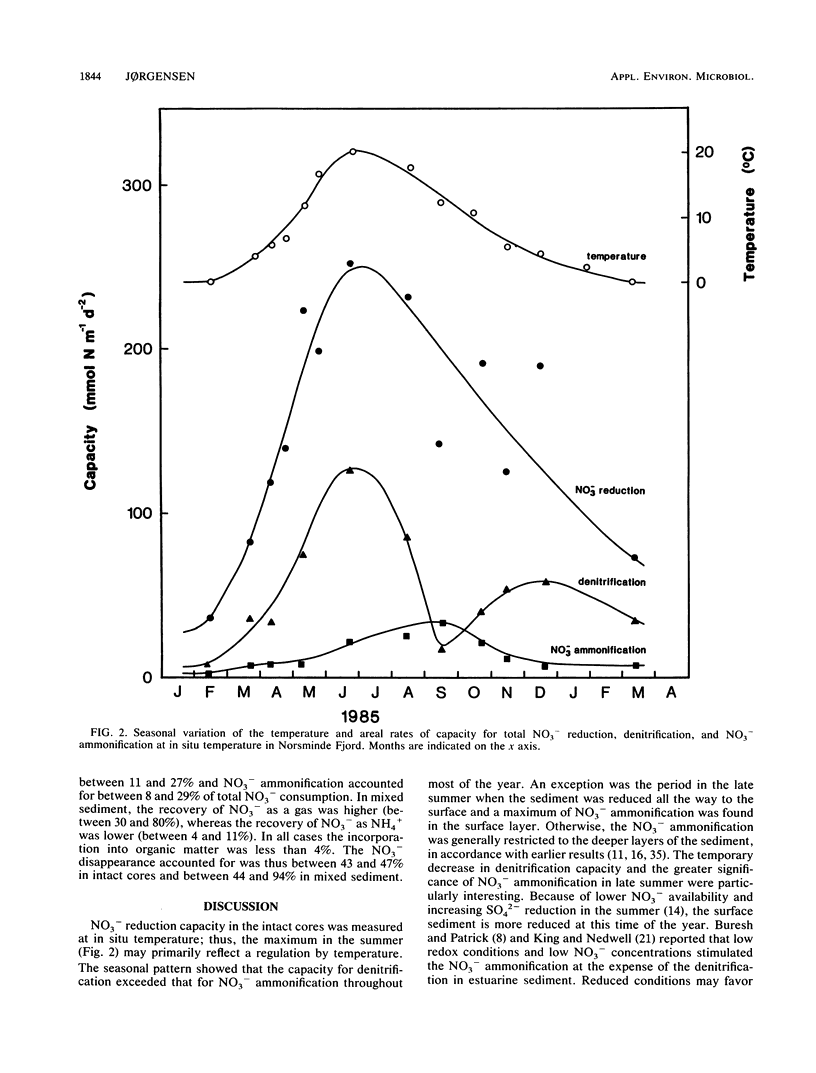
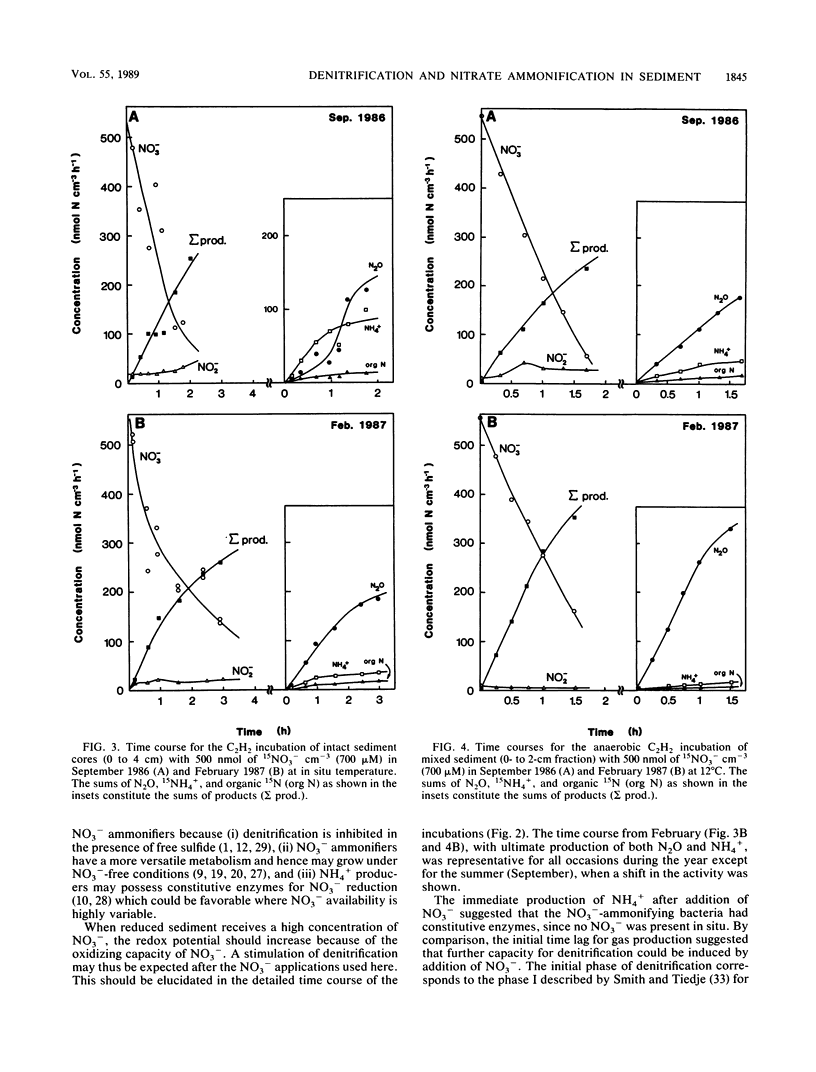
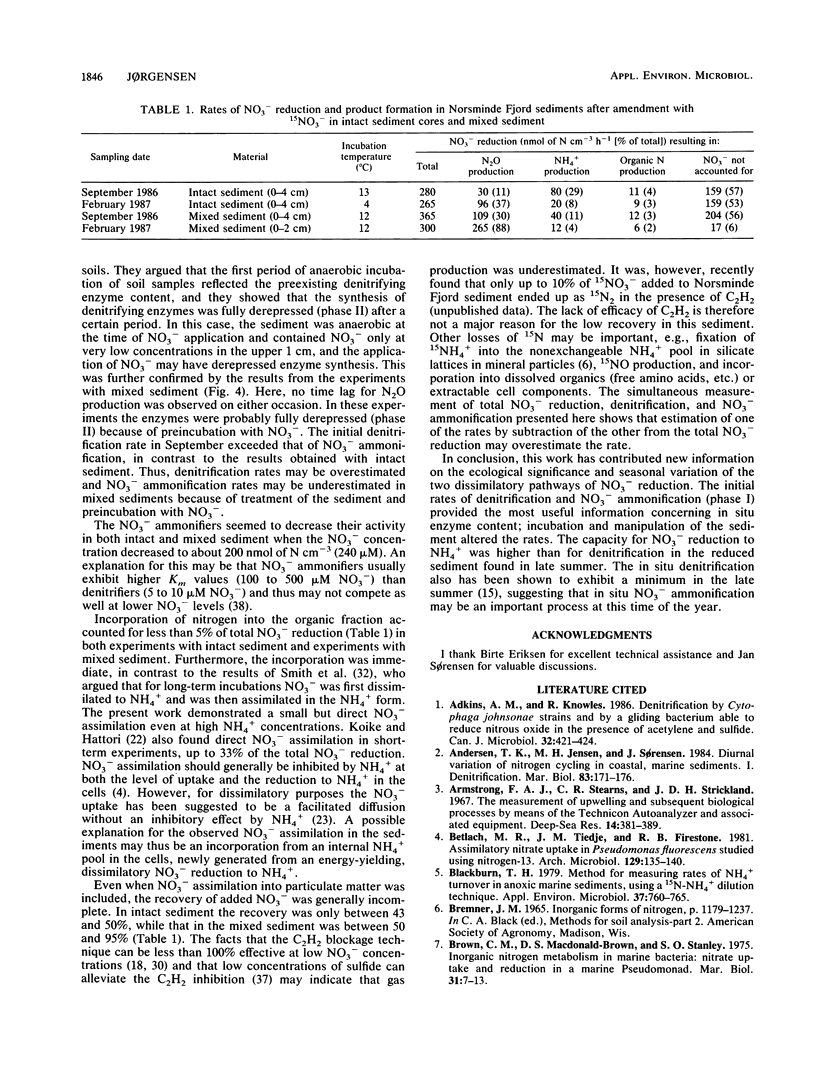
Abstract
The seasonal variation and depth distribution of the capacity for denitrification and dissimilatory NO3− reduction to NH4+ (NO3− ammonification) were studied in the upper 4 cm of the sediment of Norsminde Fjord estuary, Denmark. A combination of C2H2 inhibition and 15N isotope techniques was used in intact sediment cores in short-term incubations (maximum, 4 h). The denitrification capacity exhibited two maxima, one in the spring and one in the fall, whereas the capacity for NO3− ammonification was maximal in the late summer, when sediments were progressively reduced. The denitrification capacity was always highest in the uppermost 1 cm of the sediment and declined with depth. The NO3− ammonification was usually higher with depth, but the maximum activity in late summer was observed within the upper 1 cm. The capacity for NO3− incorporation into organic material was investigated on two occasions in intact sediment cores and accounted for less than 5% of the total NO3− reduction. Denitrification accounted for between 13 and 51% of the total NO3− reduction, and NH4+ production accounted for between 4 and 21%, depending on initial rates during the time courses. Changes of the rates during the incubation were observed in the late summer, which reflected synthesis of denitrifying enzymes. This time lag was eliminated in experiments with mixed sediment because of preincubation with NO3− and alterations of the near-environmental conditions. The initial rates obtained in intact sediment cores therefore reflect the preexisting enzyme content of the sediment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betlach M. R., Tiedje J. M., Firestone R. B. Assimilatory nitrate uptake in Pseudomonas fluorescens studied using nitrogen-13. Arch Microbiol. 1981 Apr;129(2):135–140. doi: 10.1007/BF00455349. [DOI] [PubMed] [Google Scholar]
- Blackburn T. H. Method for Measuring Rates of NH(4) Turnover in Anoxic Marine Sediments, Using a N-NH(4) Dilution Technique. Appl Environ Microbiol. 1979 Apr;37(4):760–765. doi: 10.1128/aem.37.4.760-765.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Tiedje J. M., Firestone R. B. Denitrification and dissimilatory nitrate reduction to ammonium in digested sludge. Can J Microbiol. 1981 Sep;27(9):878–885. doi: 10.1139/m81-139. [DOI] [PubMed] [Google Scholar]
- Koike I., Hattori A. Denitrification and ammonia formation in anaerobic coastal sediments. Appl Environ Microbiol. 1978 Feb;35(2):278–282. doi: 10.1128/aem.35.2.278-282.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Walter B., Hollocher T. C. Respiration-dependent proton translocation and the transport of nitrate and nitrite in Paracoccus denitrificans and other denitrifying bacteria. Biochemistry. 1978 Nov 14;17(23):5014–5019. doi: 10.1021/bi00616a024. [DOI] [PubMed] [Google Scholar]
- Mancinelli R. L., Cronin S., Hochstein L. I. The purification and properties of a cd-cytochrome nitrite reductase from Paracoccus halodenitrificans. Arch Microbiol. 1986;145:202–208. doi: 10.1007/BF00446781. [DOI] [PubMed] [Google Scholar]
- Oremland R. S., Umberger C., Culbertson C. W., Smith R. L. Denitrification in san francisco bay intertidal sediments. Appl Environ Microbiol. 1984 May;47(5):1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson M. O. Dissimilatory nitrate reduction to nitrate, nitrous oxide, and ammonium by Pseudomonas putrefaciens. Appl Environ Microbiol. 1985 Oct;50(4):812–815. doi: 10.1128/aem.50.4.812-815.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Appl Environ Microbiol. 1978 Feb;35(2):301–305. doi: 10.1128/aem.35.2.301-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Appl Environ Microbiol. 1978 Feb;35(2):301–305. doi: 10.1128/aem.35.2.301-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje J. M., Sexstone A. J., Myrold D. D., Robinson J. A. Denitrification: ecological niches, competition and survival. Antonie Van Leeuwenhoek. 1982;48(6):569–583. doi: 10.1007/BF00399542. [DOI] [PubMed] [Google Scholar]


