Abstract
Type V adenylyl cyclase (ACV) belongs to the family of Ca2+- inhibited cyclases. We have generated two soluble forms of the enzyme containing the C1 or C1a region (which lacks the C-terminal 112 amino acids) linked to the C2 domain and compared their regulation with the full-length ACV. All three forms of ACV were stimulated by the α subunit of the stimulatory G protein Gs (Gsα) and forskolin. However, the synergistic stimulation by both these activators was markedly enhanced in the soluble enzymes. Moreover, the α subunit of the inhibitory G protein Gi (Giα) inhibited all forms of the enzyme, indicating that the regions for Gsα and Giα interaction are preserved in the soluble forms. Ca2+ inhibited forskolin-stimulated adenylyl cyclase (AC) activity of the full-length and C1-C2 forms of ACV but did not alter the activity of the C1a-C2 form. Maximal stimulation of AC activity by combination of Gsα and forskolin obliterated the Ca2+-mediated inhibition of the full-length and C1-C2 forms of ACV. In 45Ca2+ overlay experiments, the C1-C2 but not the C1a-C2 soluble ACV bound Ca2+. Moreover, proteins corresponding to the C1a and C2 domains did not bind calcium. On the other hand, the proteins corresponding to C1 and its C-terminal 112 amino acids (C1b) bound 45Ca2+. To our knowledge, this is the first report of nonchimeric soluble forms of AC in which regulation by Gsα and Giα is preserved. Moreover, we demonstrate that the 112 amino acid C1b region of ACV is responsible for the binding of Ca2+ and inhibition of enzyme activity.
Adenylyl cyclase (AC; E.C. 4.6.1.1) is the enzyme that converts ATP to cAMP. To date, nine distinct isoforms of mammalian AC (designated I to IX) and two splice variants of the type VIII enzyme have been cloned and characterized (1–13). Because of the diversity in their regulation, the various AC isoforms may be broadly divided into five groups. Thus, group one consists of the type I, III, and VIII AC and are stimulated by calcium and calmodulin (2, 4, 10, 13, 14). The type II and IV AC constitute group two and are stimulated by βγ subunits of the heterotrimeric G proteins provided that the active α subunit of the stimulatory G protein (Gsα) is also present (3, 5, 15). The third group contains type II and type VII isoforms, which are phosphorylated and activated by protein kinase C (11, 16). Type V and VI AC, which are inhibited by calcium (6–9), form the fourth group. Finally, the recently characterized ACIX (originally named type X) is regulated by calcineurin (12), and because of this unique feature, it is classified in the fifth group. Despite the differences in regulation of the various AC isoforms described above, the one common regulatory feature shared is that all isoforms are stimulated by the active, GTP-bound, Gsα (for review, see refs. 17 and 18). On the other hand, the α subunit of the inhibitory G protein Gi (Giα) inhibits ACV and ACVI but not ACI and ACII (18); the type I enzyme is inhibited by G protein βγ subunits (19).
The predicted structure of all AC isoforms essentially has an N-terminal cytosolic tail and two regions each consisting of six membrane spanning domains that separate two major cytosolic loops, C1 and C2 (refs. 1, 17, and 18; see schematic in Fig. 1A). Recent studies from Gilman and coworkers have demonstrated that AC activity can be reconstituted by the expression of the two major cytosolic regions of type I and II enzyme that are either joined together by a linker (20) or expressed separately and then mixed together (21). Thus, the engineered soluble forms of AC not containing the membrane spanning regions can be utilized to investigate the regions on the molecule that are important for its regulation by various modulators. By using ACV as a representative of the Ca2+-inhibited isoforms of the enzyme, in this communication, we report the construction, expression, and partial purification of, to our knowledge, the first nonchimeric forms of AC that are stimulated by Gsα and inhibited by Giα. Our results show that the synergistic stimulation by Gsα plus forskolin is markedly enhanced in the soluble forms of ACV as compared with the full-length form of the enzyme. Moreover, we demonstrate that a 112-amino acid region in the C terminus of the first large cytosolic loop (C1 region) is important for the binding of calcium and conferring calcium sensitivity to ACV.
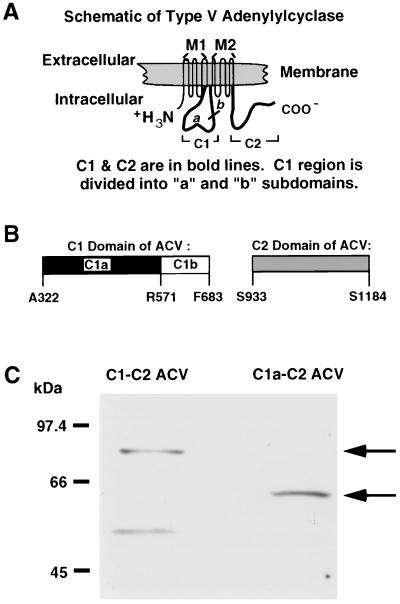
Figure 1.
Schematic representation of ACV, its major subdomains, and immunoblot of the soluble C1-C2 and C1a-C2 forms of ACV. (A) Schematic diagram of the full-length ACV. The location of the major cytosolic regions C1 and C2 is shown in reference to the whole molecule. The M1 and M2 regions, each of which spans the membrane six times, are also denoted. (B) Schematic representation of the C1 and C2 domains. The amino acid residues that define the C1a and C1b subdomains of the C1 region are shown. (C) Western blot analysis of the two soluble forms of ACV, C1-C2 and C1a-C2. The two soluble forms of ACV were expressed in TP2000 strain of E. coli. Supernatants from cell lysates were applied to Ni-NTA chromatography and partially purified C1-C2 ACV and C1a-C2 ACV were obtained by elution with imidazole. The proteins (1.5 μg per lane) were separated on 7.5% polyacrylamide gels and Western blot analysis was performed with Anti-Xpress antibody, which recognizes a sequence in the N-terminal tag of each protein. The low molecular mass band (≈50 kDa) in C1-C2 ACV represents a degradation product of the C1-C2 protein.
METHODS
Constructs for Expression of Recombinant Proteins.
The vector pTrcHisB (Invitrogen) was used for expression of the soluble forms of canine ACV in Escherichia coli. The canine ACV cDNA (6) was obtained from Yoshihiro Ishikawa (Brigham & Women’s Hospital, Harvard Medical School, Boston). The approach employed was essentially similar to that described by Tang and Gilman (20). To generate soluble ACV constructs encoding the C1 or C1a region linked to the C2 domain in-frame with the hexahistidine tag, the C2 region (amino acid residues 933-1184 of ACV; see Fig. 1 for schematic) was amplified by PCR using ACV cDNA (6) and cloned into the BglII and HindIII sites of plasmid pTrcHisB. The resulting plasmid was cut with BamHI and BglII and a PCR product encoding either the C1a (amino acid residues 322–571) or the whole C1 (amino acid residues 322–683) region was inserted. The oligonucleotides corresponding to the 3′ end of C1 or C1a regions introduced a linker of 14 amino acids [sequence, AAAGGM(PPAAAGGM)2] between the C1 (or C1a) and the C2 region.
By using approaches similar to those described above for the soluble forms of ACV, cDNAs corresponding to the C1a, C1, C2, and C1b regions of ACV (Fig. 1B) were also cloned into the plasmid pTrcHisB. All of the expression constructs were sequenced by using Sequenase (United States Biochemical) kit to confirm the correct sequences.
Expression of Soluble AC and Its Subdomains.
The TP2000 strain of E. coli, which does not express AC (22, 23), was used. Cells transformed with the plasmid constructs encoding C1-C2 or C1a-C2 forms of ACV were grown in Luria’s broth containing ampicillin (50 μg/ml) at 37°C until they reached an OD600 of 0.4. Expression of the proteins was induced by addition of isopropyl β-d-thiogalactoside (0.1 mM) and chloramphenicol (1 μg/ml) and incubation of the cells for 15 h at room temparature. Supernatants from bacterial cells were obtained by the methods described by Tang and Gilman (20). Protein concentration was determined by using Bradford reagent (Bio-Rad) and BSA as standard. To confirm expression, proteins in supernatants of cell lysates were separated on 7.5% polyacrylamide gels and immunoblots were performed with Anti-Xpress antibody (Invitrogen), which recognizes the epitope in the N terminus of the soluble ACV constructs, and the enhanced chemiluminescence system (Pierce).
Expression of the ACV subdomains C1, C1a, C2, and C1b was also achieved by similar methods. The bacterial cell lysates containing the C1, C1a, and C2 subdomains were applied to a nickel nitrilotriacetate-agarose (Ni-NTA agarose, Qiagen, Chatsworth, CA) column and partially purified proteins were eluted with an imidazole gradient as described by Whisnant et al. (21). Similarly, the soluble forms of ACV (C1-C2 and C1a-C2) were also partially purified by applying supernatants of bacterial cell lysates to Ni-NTA agarose chromatography. As demonstrated in Fig. 1C, the partially purified soluble forms of ACV containing the C1/C2 domains and the C1a/C2 regions migrated as proteins of 75.1 kDa and 61.9 kDa, respectively.
Expression of ACV in Sf9 cells.
Sf9 cells were infected with recombinant baculovirus derived from ACV cDNA cloned into plasmid p2Bac. Sixty hours after infection, the cells were harvested in PBS containing 3 mM benzamidine and other protease inhibitors as described by Taussig et al. (19). The cells were lysed in 25 mM Hepes, pH 7.4/1 mM EGTA/10% sucrose with protease inhibitors and aliquots were stored at −80°C until use.
AC Activity Assays.
Enzyme activity was assayed in 150 μl for 15 min at room temperature as described (24). Constitutively active Gsα (Q213L mutant) was expressed and purified as described (24). In some experiments, purified wild-type Gsα was bound to guanosine 5′-[γ-thio]triphosphate (GTP[γS]) as described (24) and used to activate AC. GTP[γS]-bound Giα1 was the generous gift from Ronald Taussig (University of Michigan, Ann Arbor, MI). The free-Ca2+ concentrations in the assay mixture were determined by using the Calcium Green-5N dye (25) and Mg2+-containing Ca2+ standards purchased from Molecular Probes.
45Ca2+ Overlay Assays.
After heating at 80°C for 5 min, partially purified proteins corresponding to C1-C2 ACV, C1a-C2 ACV, C1, C1a, and C2 domains (each at 1.5 μg) were applied to nitrocellulose using a slot-blot apparatus. In the case of protein corresponding to the C1b region of ACV, supernatant from lysates of TP2000 cells (10 μg of protein) induced to express the protein was applied to the nitrocellulose membranes; the untransfected cells were used to generate control lysate not containing C1b protein. Purified calmodulin (0.2 μg) and BSA (4 μg) were used as positive and negative controls, respectively. Binding of 45Ca2+ to proteins on nitrocellulose was determined as described by Marayuma et al. (26).
RESULTS AND DISCUSSION
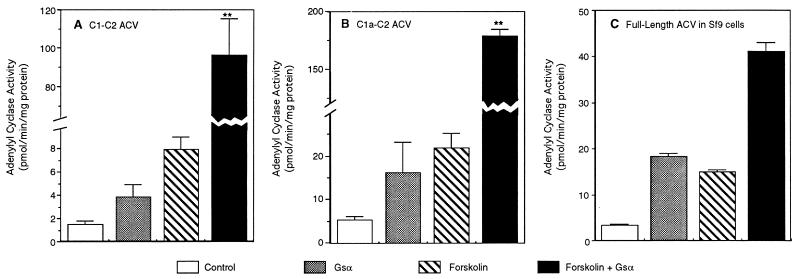
By using membranes of Sf9 cells expressing the full-length ACV and supernatants of bacterial lysates containing the soluble forms of ACV, experiments were performed to determine whether all three forms of the enzyme were regulated by activated Gsα and forskolin. As demonstrated by data in Fig. 2, all three forms of AC were stimulated by Gsα and forskolin. As described by McHugh-Sutkowski et al. (27) for the full-length ACV, when Gsα and forskolin were added together, the activity of the full-length enzyme in Sf9 cells was only modestly increased over the sum of the stimulation observed with forskolin and Gsα alone (Fig. 2C). On the other hand, in the presence of Gsα and forskolin the activity of both of the soluble forms of ACV was synergistically stimulated to levels that were at least 10-fold greater than either of the two activators alone (Fig. 2 A and B; note broken scale on abscissa). These data demonstrate that as described by Tang and Gilman (20) for the chimeric soluble AC containing the C1a region of ACI and the C2 region of ACII, the expression of comparable regions of ACV is sufficient to observe stimulation of enzyme activity by Gsα. Moreover, the data presented in Fig. 2 show that synergistic stimulation by Gsα and forskolin of ACV can be markedly augmented if the N terminus and both of the transmembrane regions of the enzyme are deleted (compare Fig. 2 A and B with C).
Figure 2.
Forskolin and Gsα stimulate the activity of the soluble and full-length forms of ACV. (A) C1-C2 form of ACV. (B) C1a-C2 form of ACV. (C) Full-length ACV expressed in Sf9 cells. The supernatant (100 μg of protein) of bacterial cell lysates containing the soluble forms of ACV or membranes from Sf9 cells (20 μg of protein) expressing the full-length enzyme were assayed for AC activity in the absence and presence of forskolin (100 μM) and Gsα (100 nM). The combined stimulation with forskolin and Gsα was also monitored. Controls presented are basal activities. Values are the mean ± SEM of four determinations. Note the broken scale in A and B to accommodate the combined forskolin + Gsα-stimulated activity. Statistical significance between the observed activities in the presence of Gsα plus forskolin and the sum of the activities observed with each agent separately was assessed by Student’s unpaired t test analysis. ∗∗, P < 0.003.
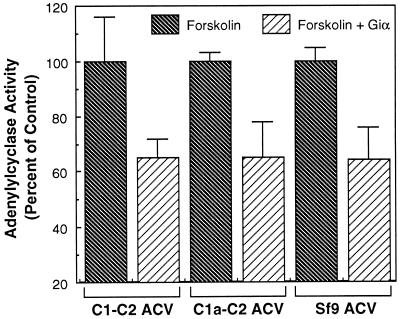
We next investigated the ability of Giα to inhibit the activity of the three forms of ACV. In these studies, the ability of Giα1 to inhibit forskolin-stimulated activity of the three forms of enzyme was investigated. As demonstrated in Fig. 3, Giα1 inhibited the activity of all three forms of the enzyme to the same extent. Thus, the ability of Giα to inhibit (Fig. 3) and Gsα to stimulate activity of ACV (Fig. 2) is preserved in the C1a-C2 and C1-C2 soluble forms of the enzyme. In this respect, to our knowledge, this is the first demonstration of any soluble form of mammalian AC that can be regulated by both Gsα and Giα. Moreover, our data demonstrate that deletion of the N terminus and the two sets of transmembrane spanning regions in ACV does not alter the ability of Giα to inhibit the enzyme. Similarly, the deletion of 112 amino acids (C1b region) in the C terminus of C1 domain does not affect inhibition by Giα.
Figure 3.
Giα1 inhibits the activity of different forms of ACV. Supernatants of cell lysates containing soluble forms of ACV or membranes from Sf9 cells expressing full-length ACV were assayed for AC activity in the presence of forskolin (100 μM) in the presence or absence of Giα1 (1 μM). Forskolin-stimulated activity for all forms of ACV is presented as 100% to facilitate comparisons. The data are from three determinations (mean ± SEM).
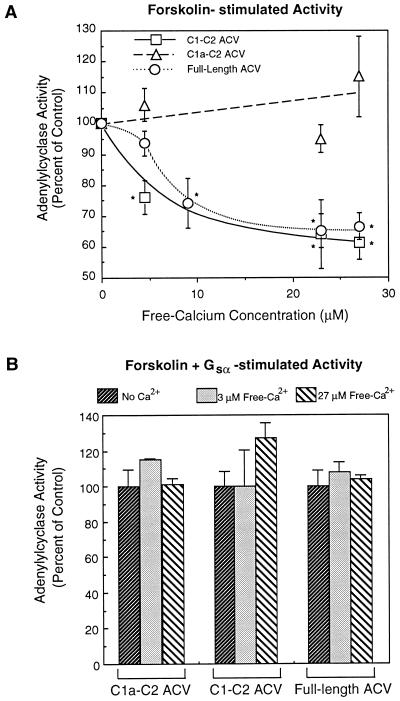
To investigate the modulation of ACV by calcium, activity of the full-length and two soluble forms of the enzyme were monitored at different calcium concentrations. The basal activities of the different ACV forms are low (Fig. 2) and, therefore, modulation by calcium of basal activity is difficult to monitor. However, as demonstrated by data in Fig. 4A, the forskolin-stimulated activity of the full-length and C1-C2 soluble form of ACV were similarly inhibited by calcium. Half-maximal inhibition of C1-C2 and full-length ACV was observed at free-Ca2+ concentrations of 5 μM and 7 μM, respectively (Fig. 4A). On the other hand, the activity of the shorter soluble form of ACV, C1a-C2, was not inhibited by free-Ca2+ concentrations of up to 27 μM (Fig. 4A). In our studies, the full-length and C1-C2 forms of ACV are inhibited by Ca2+ concentrations lower than those reported by Ishikawa et al. (6) for the full-length ACV. This difference is most likely explained by the fact that Ishikawa et al. (6) did not determine the free-Ca2+ concentrations in their assay mixture. However, the inhibition of ACV (full-length and C1-C2 forms) by Ca2+ is observed at concentrations of Ca2+ that are higher than those reported by Yoshimura and Cooper (7) for the type VI enzyme. This difference in sensitivity of ACV and ACVI to inhibition by Ca2+ is probably related to the subtle difference in the Ca2+ binding domains of the two proteins (discussed below). It is noteworthy that the effects of Ca2+ are not due to some nonspecific effect since at the concentrations tested, the activity of forskolin-stimulated C1a-C2 form of ACV was not altered (Fig. 4A). This latter contention is further supported by the observation that when the activity of the various forms of ACV was maximally stimulated by addition of forskolin and Gsα, Ca2+ did not inhibit enzyme activity at the highest concentration tested (Fig. 4B). Since the only difference between the two soluble forms of ACV is that the shorter form (C1a-C2) lacks the 112-amino acid sequence at the C terminus of the C1 region (Fig. 1B), the data in Fig. 4A demonstrate that the 112-amino acid C1b region (Arg-571 to Phe-683) is important for inhibition of enzyme activity by calcium.
Figure 4.
Ca2+ differentially inhibits the activity of the different forms of ACV. (A) The forskolin (100 μM)-stimulated activities of the full-length ACV in Sf9 cell membranes, and the two soluble forms of ACV, C1-C2 and C1a-C2, in supernatants of bacterial cell lysates in the presence of various Ca2+ concentrations were monitored as described in Fig. 1 and Methods. Free-Ca2+ concentrations were determined using Calcium Green-5N dye. (B) Same as A, except that the activities of the different forms of ACV were maximally stimulated by the combination of Gsα (100 nM) and forskolin (100 μM). Values are the mean ± SEM of four determinations. Significant differences were assessed by Student’s unpaired t test analyses. ∗, P < 0.003 (as compared with controls without Ca2+).
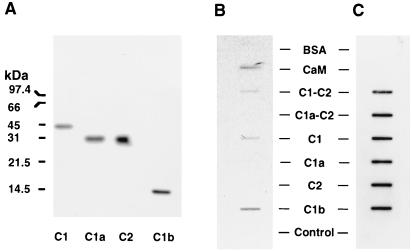
While the data with the soluble forms of ACV demonstrate that the C-terminal region of C1 domain (C1b region) is important for the inhibitory effects of calcium (Fig. 4), these data do not indicate whether or not the C1b region is indeed responsible for the binding of calcium. Therefore, additional experiments were performed in which the ability of 45Ca2+ to bind to partially purified soluble forms of ACV or subdomains of these proteins (C1, C1a, and C2 regions), as well as purified calmodulin (positive control) and BSA (negative control), was assessed. By using lysates of bacterial cells either transfected or not transfected with plasmid encoding the C1b region, the ability of 45Ca2+ to bind the 112-amino acid C1b region was also tested. As demonstrated in Fig. 5A, the hexahistidyl-tagged forms of C1, C1a, C2, and C1b regions of ACV migrate as proteins of 45.2 kDa, 32 kDa, 32.2 kDa, and 16.7 kDa, respectively (Fig. 5A). To assess the binding of Ca2+ to the two soluble forms of ACV and various domains of these enzymes, the partially purified proteins corresponding to C1-C2 ACV, C1a-C2 ACV, C1, C1a, and C2 regions of ACV, or bacterial cell lysate containing C1b protein were applied to nitrocellulose membrane along with purified calmodulin and BSA as controls. The nitrocellulose membrane was then exposed to 45Ca2+, and after washing, bound calcium was detected by autoradiography. As demonstrated by data in Fig. 5B, the C1-C2 form of ACV, but not the C1a-C2 form of the soluble enzyme, bound calcium. Moreover, neither the C1a nor the C2 domains of ACV bound calcium. However, the C1 and the C1b regions of ACV bound calcium. As expected, calmodulin, but not BSA, also bound 45Ca2+ (Fig. 5B). Additionally, the supernatant from lysates of cells not expressing C1b protein also did not bind 45Ca2+ (Fig. 5B) These data demonstrate that the C-terminal 112 amino acids (C1b region) in C1, which are missing in the C1a region, are responsible for the binding of calcium. Most importantly, the C1a-C2 soluble form of ACV, which is not inhibited by calcium, does not bind 45Ca2+ since the C1b region is not present in this protein (Fig. 5B). Notably, the lack of 45Ca2+ binding to C1a-C2 ACV or its subdomains C1a and C2 is not the result of differences in the amount of protein bound to the nitrocellulose since as determined by immunoblotting of the same membrane with Anti-Xpress antibody, which recognizes an N-terminal tag on each of the recombinant proteins, showed that all proteins were present in approximately equal amounts (Fig. 5C).
Figure 5.
Binding of 45Ca2+ to C1-C2 and C1a-C2 forms of ACV and various subdomains of these enzymes. (A) Western blot analyses of partially pure C1, C2, and C1a regions of ACV and the presence of C1b region in supernatant of lysates from bacteria induced to express the protein. Partially purified C1, C1a, and C2 proteins (1.5 μg per lane) or supernatant from bacterial lysate containing the protein corresponding to C1b region (10 μg per lane) were subjected to SDS/PAGE in 15% gels and after transfer to nitrocellulose detected with the Anti-Xpress antibody, which recognizes the N-terminal tag in these proteins. (B) Binding of 45Ca2+ to the C1-C2 form of ACV and its subdomains C1 and C1b. The partially purified proteins corresponding to C1-C2 ACV, C1a-C2 ACV, and their subdomains C1, C1a, and C2 (each at 1.5 μg) were applied to nitrocellulose membrane with a slot-blot apparatus. Calmodulin (0.2 μg), BSA (2 μg), and supernatant of lysates from bacteria (10 μg of protein) either expressing C1b or not expressing C1b (control) were similarly applied to the nitrocellulose. Control represents lysate from bacteria not expressing any ACV subdomain. Binding of 45Ca2+ was assessed. (C) The nitrocellulose membrane containing the soluble forms of ACV and their subdomains was exposed to Anti-Xpress antibody to demonstrate the presence of the various recombinant proteins. The experiments shown in B are representative of four similar experiments.
Since the inhibition of ACV and ACVI activities by calcium does not require exogenously added calmodulin (6, 7), it has been speculated that either the effects of calcium are direct or that calmodulin (or a calmodulin-like molecule) may be tightly bound to the ACV and ACVI isoforms. However, this latter possibility is unlikely since the C terminus of the C1 region of ACV can bind calcium directly, and deletion of this region in the C1a-C2 form of soluble ACV is associated with loss of calcium binding and Ca2+-mediated inhibition of activity. The C1b regions in the ACV and ACVI are similar to each other, but dissimilar in other forms of the enzyme. Because type V and VI are the only forms of AC that are inhibited by Ca2+, it would appear that the C1b region in both ACV and ACVI is responsible for binding of Ca2+ and inhibition of enzyme activity. Interestingly, the C1b region of ACI has been demonstrated to be important for the stimulatory actions of Ca2+/calmodulin on this enzyme (28) and mutation of Lys-504 to Asp within this region of ACI diminishes the ability of Ca2+/calmodulin to stimulate enzyme activity (29). Thus, it would appear that among the forms of AC that are regulated by Ca2+ or Ca2+/calmodulin the C1b region plays a critical role.
The C1b region of ACV does not contain an EF-hand motif found in proteins such as calmodulin that bind calcium (30). However, secondary structure predictions of this region by the Chou and Fasman method (31) demonstrate the presence of two loops that resemble loop 1 and loop 3 of synaptotagmin and protein kinase C-β. Thus these two loops form a C2 motif for calcium binding (32). The signature of the C2 motif includes two residues (Asp, Glu, or Asn) in loop 1 separated by 5 ± 1 amino acids and three residues (Asp, Glu, or Asn) from loop 3 that are separated by 1 and 5 ± 1 residues (32). Within the C1b region of ACV residues 621–627 (sequence, EDPKDKN) are placed within a loop, in a way similar to the aspartate residues in loop 3 of synaptotagmin (32); Asn-627 and Asp-625 are separated by one residue and Asn-627 is separated from Asp-622 or Glu-621 by 4 and 5 amino acids, respectively. Similarly, the aspartate residues in the region of residues 647–652 (sequence DARSID) of C1b region of ACV are located within a loop in positions similar to the aspartate residues in loop 1 of synaptotagmin and separated from each other by 4 amino acids (32). The secondary structure of the C1b region also predicts that the two loops containing the acidic residues that suffice for the C2 motif requirements are separated by an intermediate helix–loop–helix that would allow the two loops to be in proximity of each other. Among the 32 residues between Glu-621 and Asp-652 that form the two loops that may constitute the C2 motif, 13 residues are acidic. Most of these acidic residues are highly conserved among the ACV and ACVI forms from different species. Thus, it would appear that this 32-amino acid region in C1b that includes the the C2 calcium binding motif in ACV and ACVI from different mammalian species serves to bind calcium. Interestingly, the equivalent of Glu-621 in ACV is Asp-602 in ACVI. Since aspartate residues have a higher affinity for Ca2+ than do glutamate residues, it is possible that the C2 motif in C1b region of ACVI binds Ca2+ with a higher affinity than the similar region in ACV. This may explain the greater sensitivity of ACVI to inhibition by Ca2+ as compared with ACV (c.f. Fig. 4A and ref. 7). However, this possibility remains to be experimentally tested and forms the subject of future studies.
The significance of regulation of AC activity by calcium is underscored by the demonstration that cAMP accumulation in cells that express predominantly the type VI enzyme is reciprocally regulated by changes in intracellular calcium concentrations (33). Similarly, in cells expressing ACI, which is activated by calcium/calmodulin, alterations in cellular calcium concentrations increase cAMP accumulation (ref. 34; also for review, see 35). Since ACV and ACVI are two of the most prominent forms of AC expressed in the heart (6–9) and because oscillations in cAMP concentration in the heart have been reported to occur during cardiac contractions (36), it is tempting to speculate that the inhibition of ACV and ACVI by Ca2+ may serve as a negative feedback to inhibit enzyme activity and reduce cAMP levels during a contraction. Indeed Ca2+ has been demonstrated to inhibit catecholamine-stimulated cAMP accumulation in cardiac myocytes (37, 38). Experimental evidence also suggests that the Ca2+-inhibited ACs are colocalized with capacitative Ca2+ entry channels (39) and, therefore, may be exposed to high free-Ca2+ concentrations. This coupled with the observations that local free-Ca2+ concentrations in cells may be as high as 5–6 μM (40) would imply that ACV, which is half-maximally inhibited by free-Ca2+ concentrations of 5–7 μM, is regulated by changes in intracellular Ca2+ levels.
In summary, the data presented herein demonstrate that the expression of soluble forms of ACV consisting of C1a-C2 or C1-C2 domains reconstitutes enzyme activity that can be stimulated by Gsα and forskolin. Since the synergistic activation of the soluble forms of ACV is markedly enhanced as compared with the full-length enzyme, it would appear that the deletion of N terminus and/or transmembrane regions allows a greater synergism between the activators. Likewise, because Giα can inhibit both the C1a-C2 and C1-C2 forms of the enzyme, the data suggest that the Giα interaction site(s) is(are) located within the C1a and C2 regions. Moreover, our data demonstrate that the C1b region of ACV binds calcium and that deletion of this region in the C1a-C2 forms of soluble ACV obliterates the inhibition of the enzyme by calcium. Since the C1b region and most importantly, the acidic residues that may be responsible for binding Ca2+ are conserved in ACVI, which is also inhibited by calcium, it would appear that this region in the calcium-inhibited forms of AC is important for the actions of Ca2+.
Acknowledgments
We thank the following individuals for providing the various reagents: Dr. Yoshihiro Ishikawa for the canine ACV cDNA, Dr. Ronald Taussig for the Giα1, Dr. W.-J. Tang for the TP2000 strain of E. coli, and Dr. Alfred G. Gilman for the Gsα cDNA. The help of Ms. Michelle Steffen in the cloning of the C1b region of ACV in vector pTrcHisB is also acknowledged. This research was supported by National Institutes of Health Grant HL48308 and postdoctoral fellowships from the American Heart Association, Tennessee Affiliate to K.S. and A.J.B.
ABBREVIATIONS
- AC
adenylyl cyclase (roman numerals after AC, e.g., ACV, depict isoform type of AC)
- Gs and Gi
stimulatory and inhibitory GTP-binding proteins of AC, respectively
- Gsα
α subunit of Gs
- Giα
α subunit of Gi
- Ni-NTA
nickel nitrilotriacetate
References
- 1.Krupinski J, Coussen F, Bakalyar H A, Tang W-J, Feinstein P G, Orth K, Slaughter C, Reed R, Gilman A G. Science. 1989;244:1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- 2.Tang W-J, Gilman A G. J Biol Chem. 1991;266:8595–8603. [PubMed] [Google Scholar]
- 3.Feinstein P G, Schrader K A, Bakalyar H A, Tang W-J, Krupinski J, Gilman A G. Proc Natl Acad Sci USA. 1991;88:10173–10177. doi: 10.1073/pnas.88.22.10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakalyar H A, Reed R R. Science. 1990;250:1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- 5.Gao B, Gilman A G. Proc Natl Acad Sci USA. 1991;88:10178–10182. doi: 10.1073/pnas.88.22.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa Y, Shuichi K, Chen L, Halnon N J, Kawabe J-i, Homcy C. J Biol Chem. 1992;267:13553–13557. [PubMed] [Google Scholar]
- 7.Yoshimura M, Cooper D M F. Proc Natl Acad Sci USA. 1992;89:6716–6720. doi: 10.1073/pnas.89.15.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premont R T, Chen J, Ma H-W, Ponnapalli M, Iyengar R. Proc Natl Acad Sci USA. 1992;89:9809–9813. doi: 10.1073/pnas.89.20.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsushika S, Chen L, Kawabe J-I, Nilakantan R, Halnon N J, Homcy C J, Ishikawa Y. Proc Natl Acad Sci USA. 1992;89:8774–8778. doi: 10.1073/pnas.89.18.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krupinski J, Lehman T C, Frankenfield C D, Zwaagstra J C, Watson P A. J Biol Chem. 1992;267:24858–24862. [PubMed] [Google Scholar]
- 11.Watson P A, Krupinski J, Kempinski A M, Frankenfield C D. J Biol Chem. 1994;269:28893–28898. [PubMed] [Google Scholar]
- 12.Paterson J M, Smith S M, Harmar A J, Antoni F A. Biochem Biophys Res Commun. 1995;214:1000–1008. doi: 10.1006/bbrc.1995.2385. [DOI] [PubMed] [Google Scholar]
- 13.Cali J J, Parekh R S, Krupinski J. J Biol Chem. 1996;271:1089–1095. doi: 10.1074/jbc.271.2.1089. [DOI] [PubMed] [Google Scholar]
- 14.Choi E-J, Xia Z, Storm D R. Biochemistry. 1992;31:6492–6498. doi: 10.1021/bi00143a019. [DOI] [PubMed] [Google Scholar]
- 15.Tang W-J, Gilman A G. Science. 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- 16.Jacobowitz O, Iyengar R. Proc Natl Acad Sci USA. 1994;91:10630–10634. doi: 10.1073/pnas.91.22.10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyengar R. FASEB J. 1993;7:768–775. doi: 10.1096/fasebj.7.9.8330684. [DOI] [PubMed] [Google Scholar]
- 18.Taussig R, Gilman A G. J Biol Chem. 1995;270:1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Taussig R, Quarmby L M, Gilman A G. J Biol Chem. 1993;268:9–12. [PubMed] [Google Scholar]
- 20.Tang W-J, Gilman A G. Science. 1995;268:1769–1772. doi: 10.1126/science.7792604. [DOI] [PubMed] [Google Scholar]
- 21.Whisnant R E, Gilman A G, Dessauer C W. Proc Natl Acad Sci USA. 1996;93:6621–6625. doi: 10.1073/pnas.93.13.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy A, Danchin A. Mol Gen Genet. 1982;188:465–471. doi: 10.1007/BF00330050. [DOI] [PubMed] [Google Scholar]
- 23.Beauve A, Boesten B, Crasnier M, Danchin A, O’Gara F. J Bacteriol. 1990;172:2614–2621. doi: 10.1128/jb.172.5.2614-2621.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H, Seyer J M, Patel T B. Proc Natl Acad Sci USA. 1995;92:2229–2233. doi: 10.1073/pnas.92.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker I, Yao Y. Cell Calcium. 1994;15:276–288. doi: 10.1016/0143-4160(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 26.Marayuma K, Mikawa J, Ebashi S. J Biochem. 1984;95:511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- 27.McHugh-Sutkowski E, Tang W-J, Broome C W, Robbins J D, Seamon K B. Biochemistry. 1994;33:12852–12859. doi: 10.1021/bi00209a017. [DOI] [PubMed] [Google Scholar]
- 28.Levine L, Reed R R. J Biol Chem. 1995;270:7573–7579. doi: 10.1074/jbc.270.13.7573. [DOI] [PubMed] [Google Scholar]
- 29.Wu Z, Wong S T, Storm D R. J Biol Chem. 1993;268:23766–23768. [PubMed] [Google Scholar]
- 30.Strynadka N C J, James M N G. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 31.Chou P Y, Fasman G D. Biochemistry. 1974;13:222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- 32.Shao X, Davletov B A, Sutton R B, Südhof T C, Rizo J. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- 33.DeBernardi M A, Brooker G A. Proc Natl Acad Sci USA. 1996;93:4577–4582. doi: 10.1073/pnas.93.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi E-J, Wong S T, Hinds T R, Storm D R. J Biol Chem. 1992;267:12440–12442. [PubMed] [Google Scholar]
- 35.Cooper D F M, Mons N, Karpen J W. Nature (London) 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 36.Brooker G. Science. 1973;182:933–934. doi: 10.1126/science.182.4115.933. [DOI] [PubMed] [Google Scholar]
- 37.Yu H-J, Ma H, Green R D. Mol Pharmacol. 1993;44:689–693. [PubMed] [Google Scholar]
- 38.Colvin R A, Oiba J A, Allen R A. Cell Calcium. 1991;12:19–27. doi: 10.1016/0143-4160(91)90081-o. [DOI] [PubMed] [Google Scholar]
- 39.Chiono M, Mahey R, Tate G, Cooper D M F. J Biol Chem. 1995;270:1149–1155. doi: 10.1074/jbc.270.3.1149. [DOI] [PubMed] [Google Scholar]
- 40.Rizzuto R, Brini M, Murgia M, Pozzan T. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]