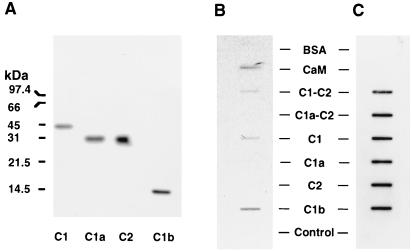
Figure 5.
Binding of 45Ca2+ to C1-C2 and C1a-C2 forms of ACV and various subdomains of these enzymes. (A) Western blot analyses of partially pure C1, C2, and C1a regions of ACV and the presence of C1b region in supernatant of lysates from bacteria induced to express the protein. Partially purified C1, C1a, and C2 proteins (1.5 μg per lane) or supernatant from bacterial lysate containing the protein corresponding to C1b region (10 μg per lane) were subjected to SDS/PAGE in 15% gels and after transfer to nitrocellulose detected with the Anti-Xpress antibody, which recognizes the N-terminal tag in these proteins. (B) Binding of 45Ca2+ to the C1-C2 form of ACV and its subdomains C1 and C1b. The partially purified proteins corresponding to C1-C2 ACV, C1a-C2 ACV, and their subdomains C1, C1a, and C2 (each at 1.5 μg) were applied to nitrocellulose membrane with a slot-blot apparatus. Calmodulin (0.2 μg), BSA (2 μg), and supernatant of lysates from bacteria (10 μg of protein) either expressing C1b or not expressing C1b (control) were similarly applied to the nitrocellulose. Control represents lysate from bacteria not expressing any ACV subdomain. Binding of 45Ca2+ was assessed. (C) The nitrocellulose membrane containing the soluble forms of ACV and their subdomains was exposed to Anti-Xpress antibody to demonstrate the presence of the various recombinant proteins. The experiments shown in B are representative of four similar experiments.