Abstract
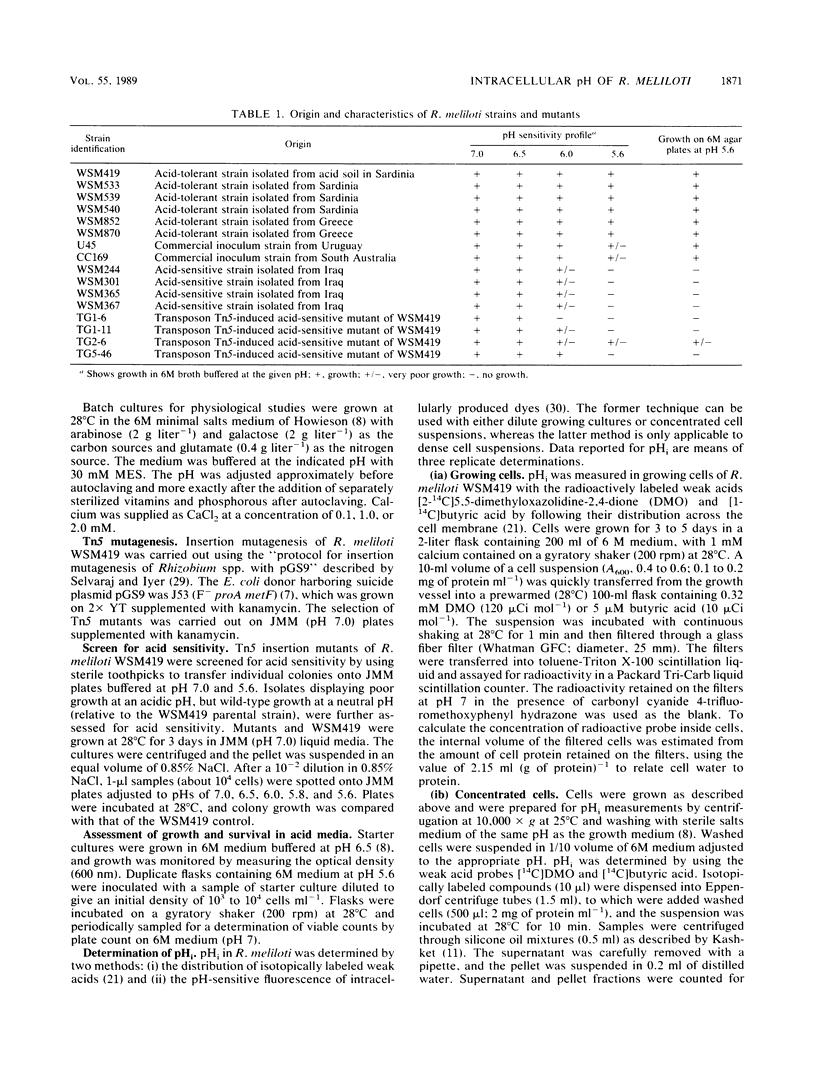
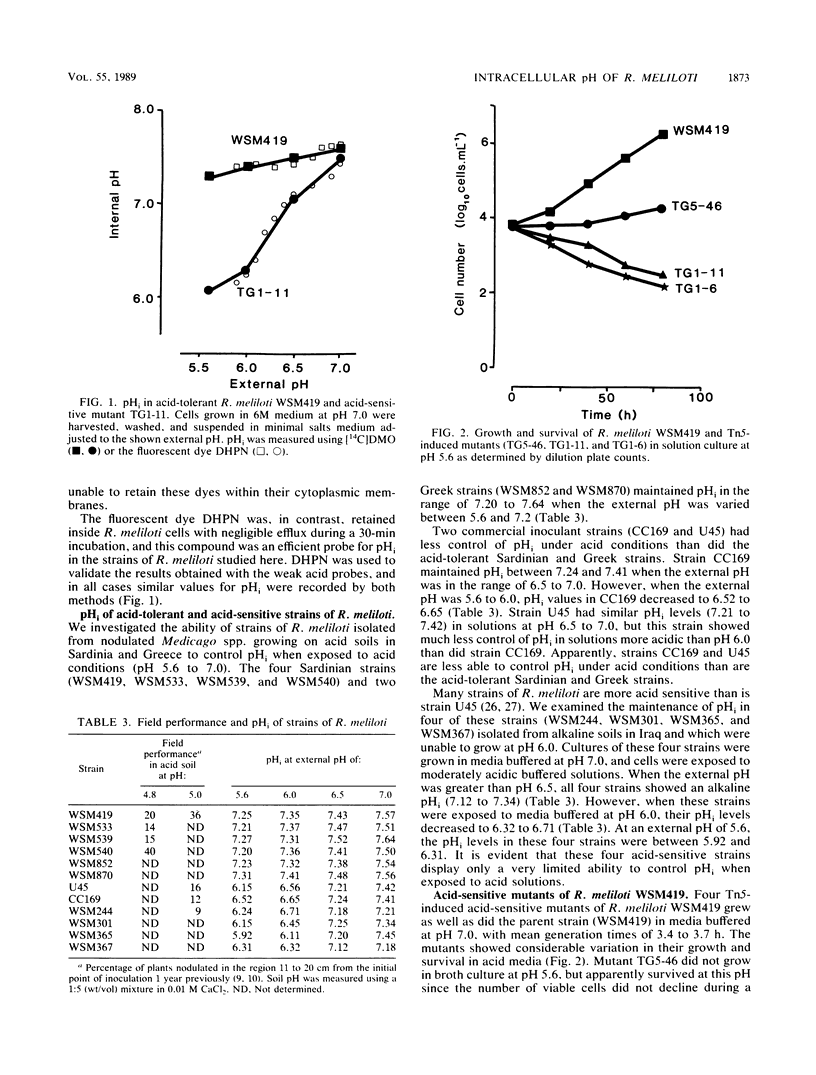
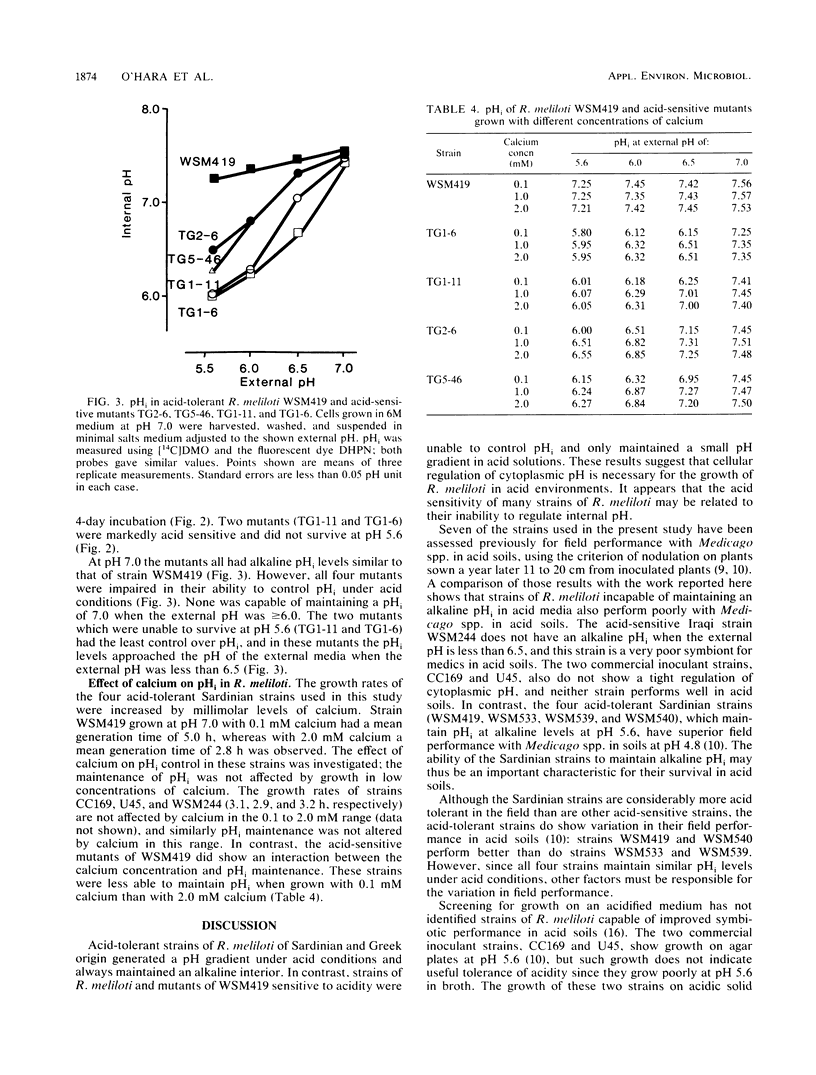
The development and function of the Rhizobium meliloti-Medicago sp. symbiosis are sensitive to soil acidity. Physiological criteria that can be measured in culture which serve to predict acid tolerance in soil would be valuable. The intracellular pH of R. meliloti was measured using either radioactively labeled weak acids (5,5-dimethyloxazolidine-2,4-dione and butyric acid) or pH-sensitive fluorescent compounds; both methods gave similar values. Six acid-tolerant strains (WSM419, WSM533, WSM539, WSM540, WSM852, and WSM870) maintained an alkaline intracellular pH when the external pH was between 5.6 and 7.2. In contrast, two Australian commercial inoculant strains (CC169 and U45) and four acid-sensitive strains from alkaline soils in Iraq (WSM244, WSM301, WSM365, and WSM367) maintained an alkaline intracellular pH when the external pH was ≥6.5, but had intracellular pH values of ≤6.8 when the external pH was ≤6.0. Four transposon Tn5-induced mutants of acid-tolerant strain WSM419, impaired in their ability to grow at pH 5.6, showed limited control over the intracellular pH. The ability to generate a large pH gradient under acid conditions may be a better indicator of acid tolerance in R. meliloti under field conditions than is growth on acidic agar plates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bhandari B., Nicholas D. J. Proton motive force in washed cells of Rhizobium japonicum and bacteroids from Glycine max. J Bacteriol. 1985 Dec;164(3):1383–1385. doi: 10.1128/jb.164.3.1383-1385.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. H+/ATP stoichiometry of cowpea Rhizobium sp. strain 32H1 cells grown under nitrogen-fixing and nitrogen-nonfixing conditions. J Bacteriol. 1984 Oct;160(1):216–221. doi: 10.1128/jb.160.1.216-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W. R factors from Providence. J Gen Microbiol. 1974 Mar;81(1):171–181. doi: 10.1099/00221287-81-1-171. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. Effects of aerobiosis and nitrogen source on the proton motive force in growing Escherichia coli and Klebsiella pneumoniae cells. J Bacteriol. 1981 Apr;146(1):377–384. doi: 10.1128/jb.146.1.377-384.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Murakami N., Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J Biol Chem. 1982 Nov 25;257(22):13246–13252. [PubMed] [Google Scholar]
- Kobayashi H., Unemoto T. Streptococcus faecalis mutants defective in regulation of cytoplasmic pH. J Bacteriol. 1980 Sep;143(3):1187–1193. doi: 10.1128/jb.143.3.1187-1193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz I., Balaban R. S. Fluorescence emission spectroscopy of 1,4-dihydroxyphthalonitrile. A method for determining intracellular pH in cultured cells. Biophys J. 1985 Sep;48(3):499–508. doi: 10.1016/S0006-3495(85)83805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowendorf H. S., Baya A. M., Alexander M. Survival of Rhizobium in Acid soils. Appl Environ Microbiol. 1981 Dec;42(6):951–957. doi: 10.1128/aem.42.6.951-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivan J. D. Mechanism of the stimulation of serine and alanine transport into isolated rat liver cells by bicarbonate ions. Biochem J. 1979 Sep 15;182(3):697–705. doi: 10.1042/bj1820697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Intracellular pH regulation in bacterial cells. Methods Enzymol. 1986;125:337–352. doi: 10.1016/s0076-6879(86)25029-6. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter E., Letellier L., Simons E. R. Fluorescence dye as monitor of internal pH in Escherichia coli cells. FEBS Lett. 1982 Mar 8;139(1):121–124. doi: 10.1016/0014-5793(82)80501-2. [DOI] [PubMed] [Google Scholar]
- Slavík J. Intracellular pH of yeast cells measured with fluorescent probes. FEBS Lett. 1982 Apr 5;140(1):22–26. doi: 10.1016/0014-5793(82)80512-7. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]



