Abstract
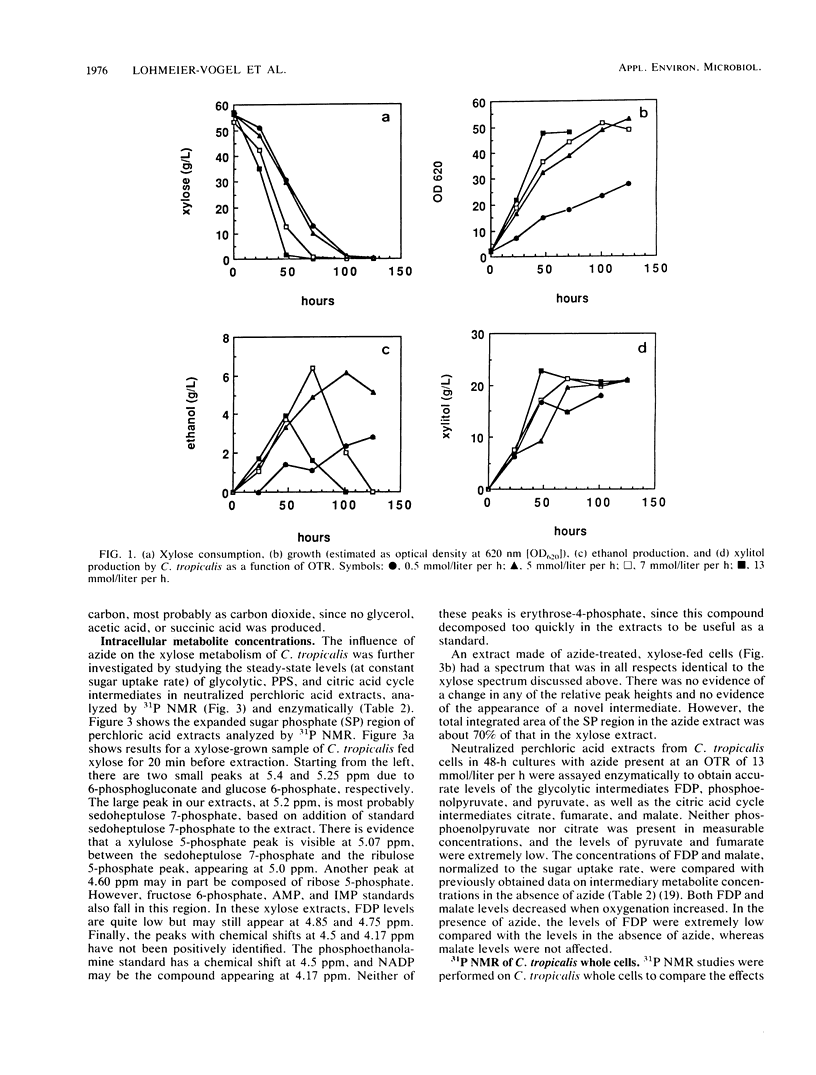
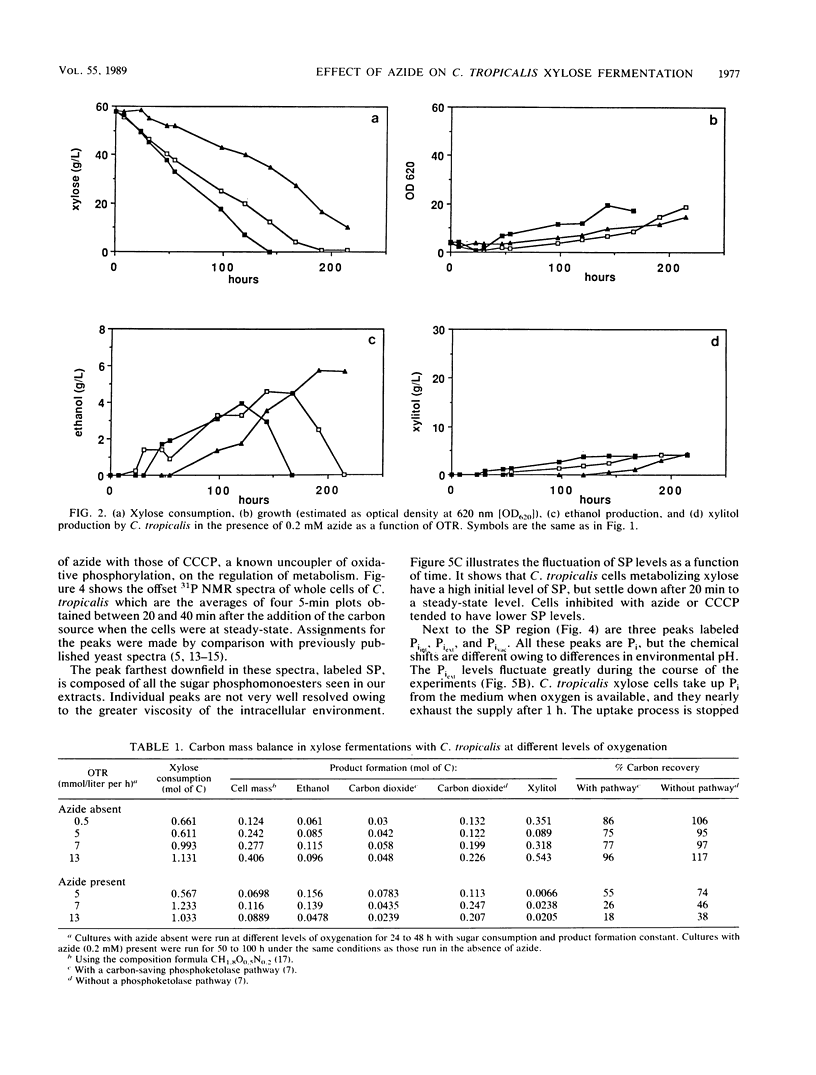
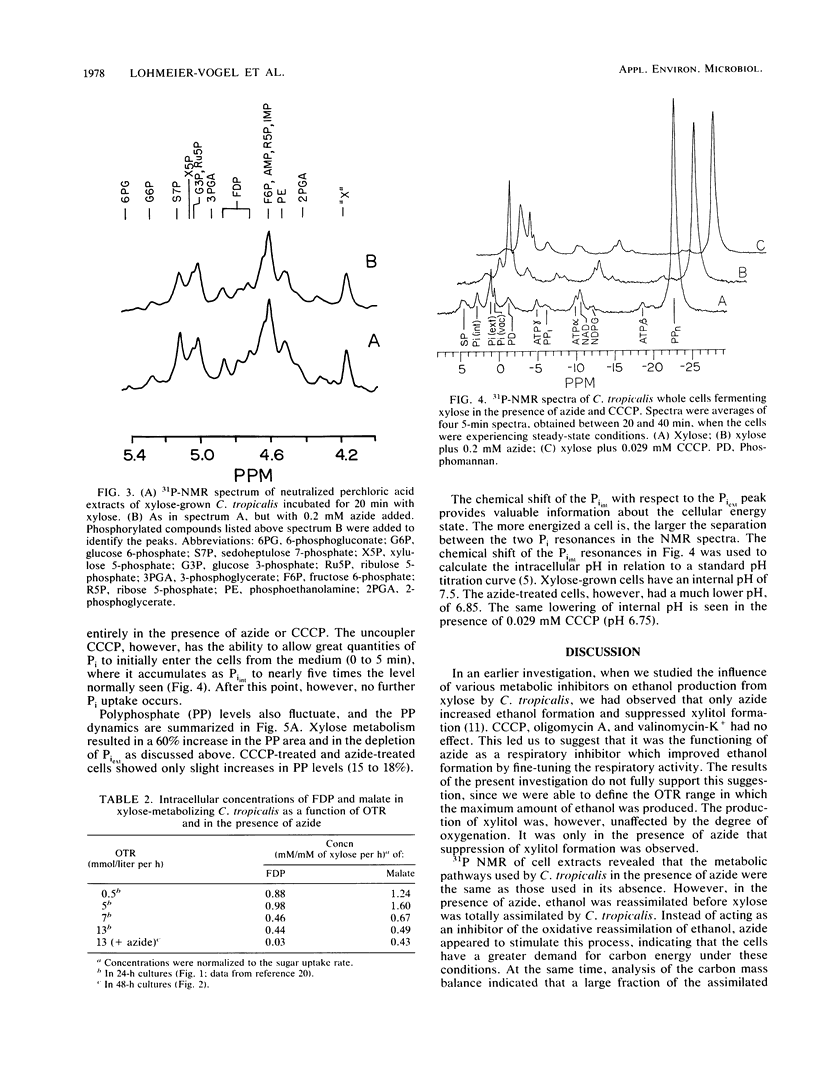
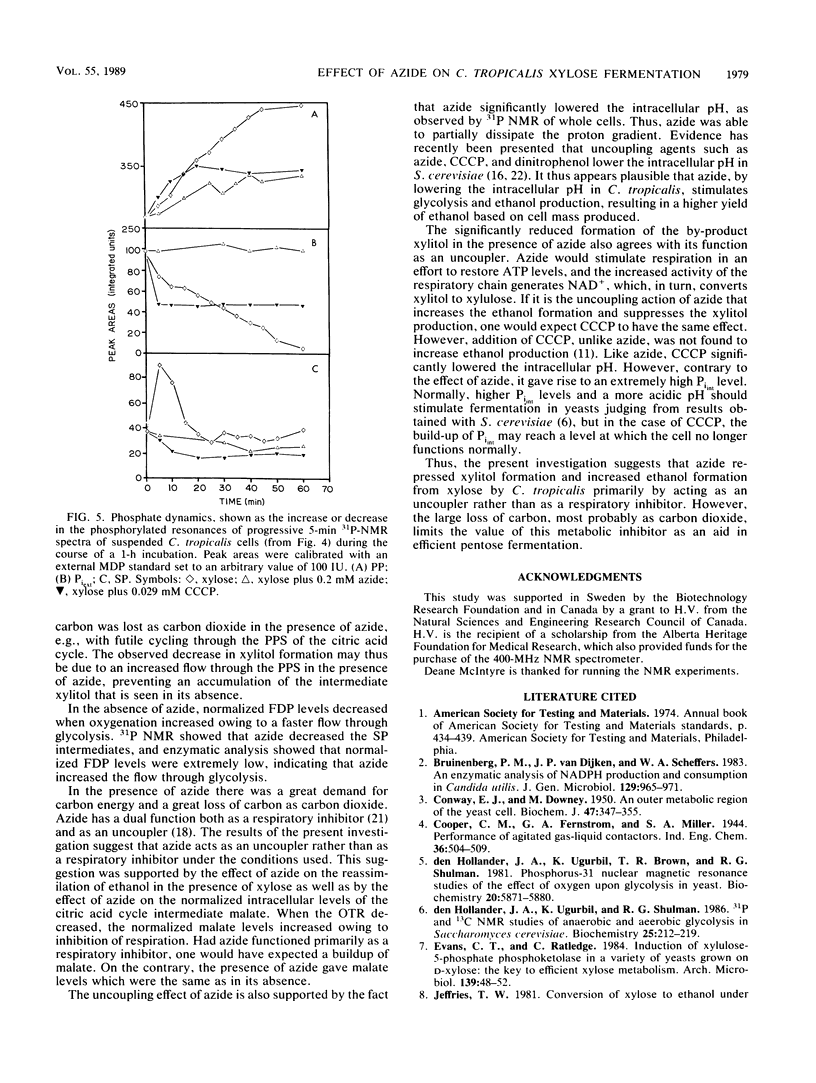
Maximal ethanol production by Candida tropicalis grown on xylose was obtained at an oxygen transfer rate of 5 to 7 mmol/liter per h. Addition of 0.2 mM azide increased the ethanol yield by a factor of 3 to 4, based on the cell mass produced, and decreased the formation of the by-product xylitol by 80%. In the presence of azide, ethanol was reassimilated before the carbon source was depleted. At all oxygenation levels studied, azide caused 25 to 60% of the carbon to be lost, most probably as carbon dioxide. Identical spectra were obtained with 31P nuclear magnetic resonance spectroscopy performed on extracts of C. tropicalis grown on xylose in the absence and presence of azide. Azide lowered the levels of sugar phosphates. Enzymatic analysis showed extremely low levels of fructose 1,6-diphosphate compared with the levels obtained in the absence of azide, while the level of malate, a citric acid cycle intermediate, was not influenced by azide. 31P nuclear magnetic resonance spectroscopy performed on xylose-grown whole cells of C. tropicalis showed that azide lowered the intracellular pH, inhibited the uptake of external Pi, and decreased the buildup of polyphosphate in relation to results with untreated cells. Similar results were obtained with the uncoupler of oxidative phosphorylation carbonyl cyanide m-chlorophenylhydrazone (CCCP), except that CCCP treatment led to extremely high levels of internal Pi. The dual effect of azide as a respiratory inhibitor and as an uncoupler is discussed with respect to the metabolism and product formation in xylose-assimilating C. tropicalis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruinenberg P. M., van Dijken J. P., Scheffers W. A. An enzymic analysis of NADPH production and consumption in Candida utilis. J Gen Microbiol. 1983 Apr;129(4):965–971. doi: 10.1099/00221287-129-4-965. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. An outer metabolic region of the yeast cell. Biochem J. 1950 Sep;47(3):347–355. doi: 10.1042/bj0470347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Scheffers W. A., Bruinenberg P. M., Kaptein R. In vivo 31P NMR studies on the role of the vacuole in phosphate metabolism in yeasts. Arch Microbiol. 1983 Jul;134(4):270–275. doi: 10.1007/BF00407801. [DOI] [PubMed] [Google Scholar]
- Noshiro A., Purwin C., Laux M., Nicolay K., Scheffers W. A., Holzer H. Mechanism of stimulation of endogenous fermentation in yeast by carbonyl cyanide m-chlorophenylhydrazone. J Biol Chem. 1987 Oct 15;262(29):14154–14157. [PubMed] [Google Scholar]
- ROTHSTEIN A., BERKE H. Endogenous alcoholic fermentation in yeast induced by 2, 4-dinitrophenol. Arch Biochem Biophys. 1952 Mar;36(1):195–201. doi: 10.1016/0003-9861(52)90390-1. [DOI] [PubMed] [Google Scholar]
- STICKLAND L. H. The Pasteur effect in normal yeast and its inhibition by various agents. Biochem J. 1956 Nov;64(3):503–515. doi: 10.1042/bj0640503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., Beullens M., Honshoven F., Hoebeeck G., Detremerie K., den Hollander J. A., Jans A. W. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: intracellular pH and the effect of membrane depolarizing compounds. J Gen Microbiol. 1987 Aug;133(8):2191–2196. doi: 10.1099/00221287-133-8-2191. [DOI] [PubMed] [Google Scholar]
- den Hollander J. A., Ugurbil K., Brown T. R., Shulman R. G. Phosphorus-31 nuclear magnetic resonance studies of the effect of oxygen upon glycolysis in yeast. Biochemistry. 1981 Sep 29;20(20):5871–5880. doi: 10.1021/bi00523a034. [DOI] [PubMed] [Google Scholar]
- den Hollander J. A., Ugurbil K., Shulman R. G. 31P and 13C NMR studies of intermediates of aerobic and anaerobic glycolysis in Saccharomyces cerevisiae. Biochemistry. 1986 Jan 14;25(1):212–219. doi: 10.1021/bi00349a030. [DOI] [PubMed] [Google Scholar]


