Abstract
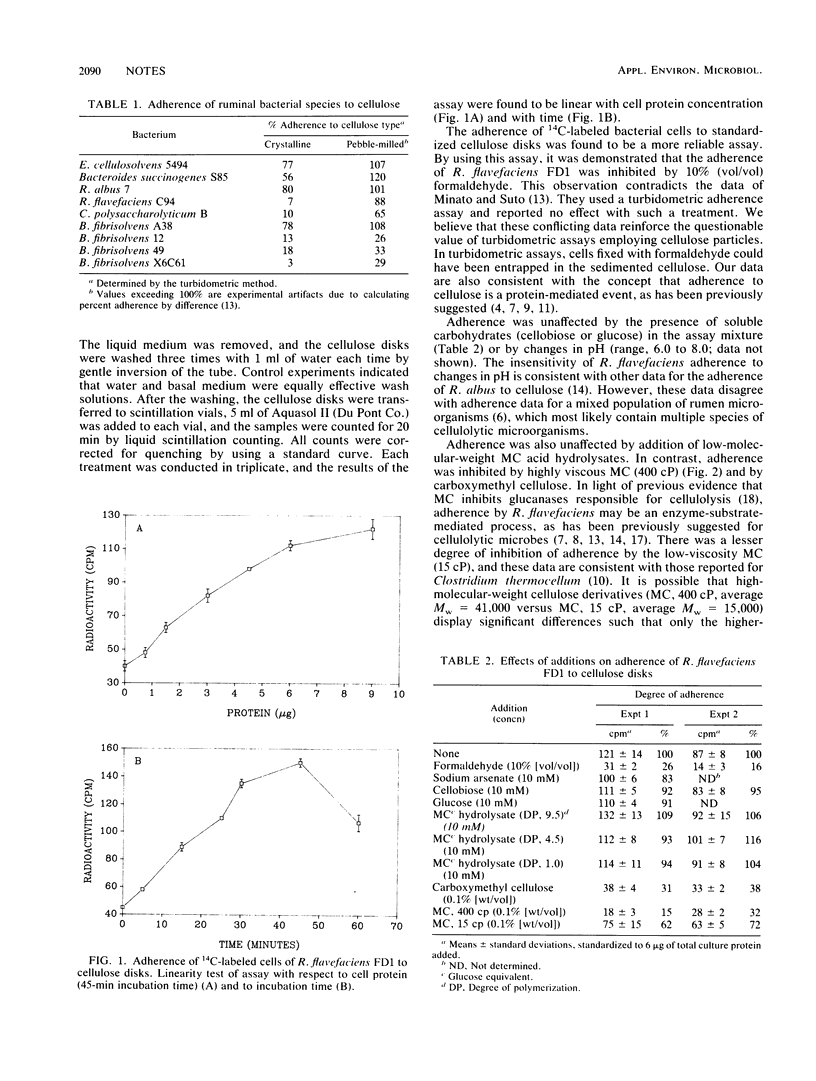
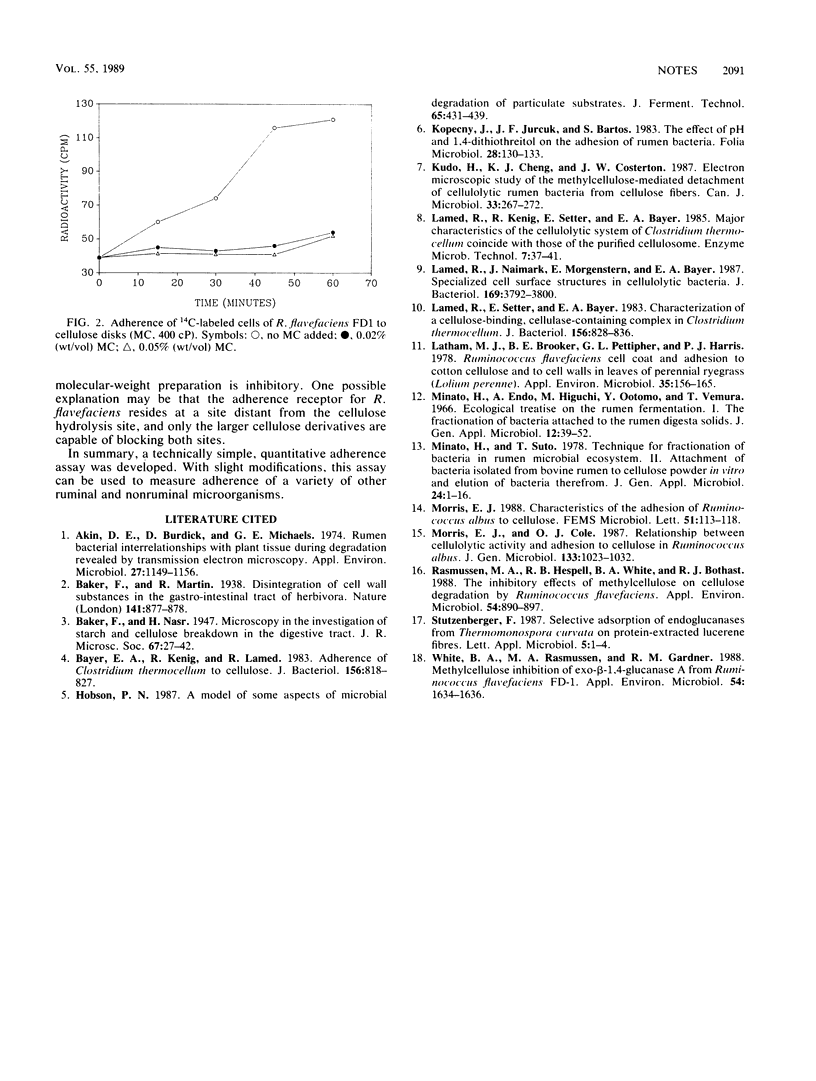
A quantitative technique suitable for the determination of adherence of ruminal bacteria to cellulose was developed. This technique employs adherence of cells to cellulose disks and alleviates the problem of nonspecific cell entrapment within cellulose particles. By using this technique, it was demonstrated that the adherence of Ruminococcus flavefaciens FD1 to cellulose was inhibited by formaldehyde, methylcellulose, and carboxymethyl cellulose. Adherence was unaffected by acid hydrolysates of methylcellulose, glucose, and cellobiose.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Kenig R., Lamed R. Adherence of Clostridium thermocellum to cellulose. J Bacteriol. 1983 Nov;156(2):818–827. doi: 10.1128/jb.156.2.818-827.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecný J., Jurcuk J. F., Bartos S. The effect of pH and 1,4-dithiothreitol on the adhesion of rumen bacteria. Folia Microbiol (Praha) 1983;28(2):130–133. doi: 10.1007/BF02877369. [DOI] [PubMed] [Google Scholar]
- Kudo H., Cheng K. J., Costerton J. W. Electron microscopic study of the methylcellulose-mediated detachment of cellulolytic rumen bacteria from cellulose fibers. Can J Microbiol. 1987 Mar;33(3):267–272. doi: 10.1139/m87-045. [DOI] [PubMed] [Google Scholar]
- Lamed R., Naimark J., Morgenstern E., Bayer E. A. Specialized cell surface structures in cellulolytic bacteria. J Bacteriol. 1987 Aug;169(8):3792–3800. doi: 10.1128/jb.169.8.3792-3800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamed R., Setter E., Bayer E. A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol. 1983 Nov;156(2):828–836. doi: 10.1128/jb.156.2.828-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Ruminococcus flavefaciens Cell Coat and Adhesion to Cotton Cellulose and to Cell Walls in Leaves of Perennial Ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jan;35(1):156–165. doi: 10.1128/aem.35.1.156-165.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. D., Bayer R. The limits of the ledger in public health promotion. Hastings Cent Rep. 1985 Dec;15(6):37–41. [PubMed] [Google Scholar]
- Rasmussen M. A., Hespell R. B., White B. A., Bothast R. J. Inhibitory Effects of Methylcellulose on Cellulose Degradation by Ruminococcus flavefaciens. Appl Environ Microbiol. 1988 Apr;54(4):890–897. doi: 10.1128/aem.54.4.890-897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Rasmussen M. A., Gardner R. M. Methylcellulose inhibition of exo-beta-1,4-glucanase A from Ruminococcus flavefaciens FD-1. Appl Environ Microbiol. 1988 Jun;54(6):1634–1636. doi: 10.1128/aem.54.6.1634-1636.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


