Abstract
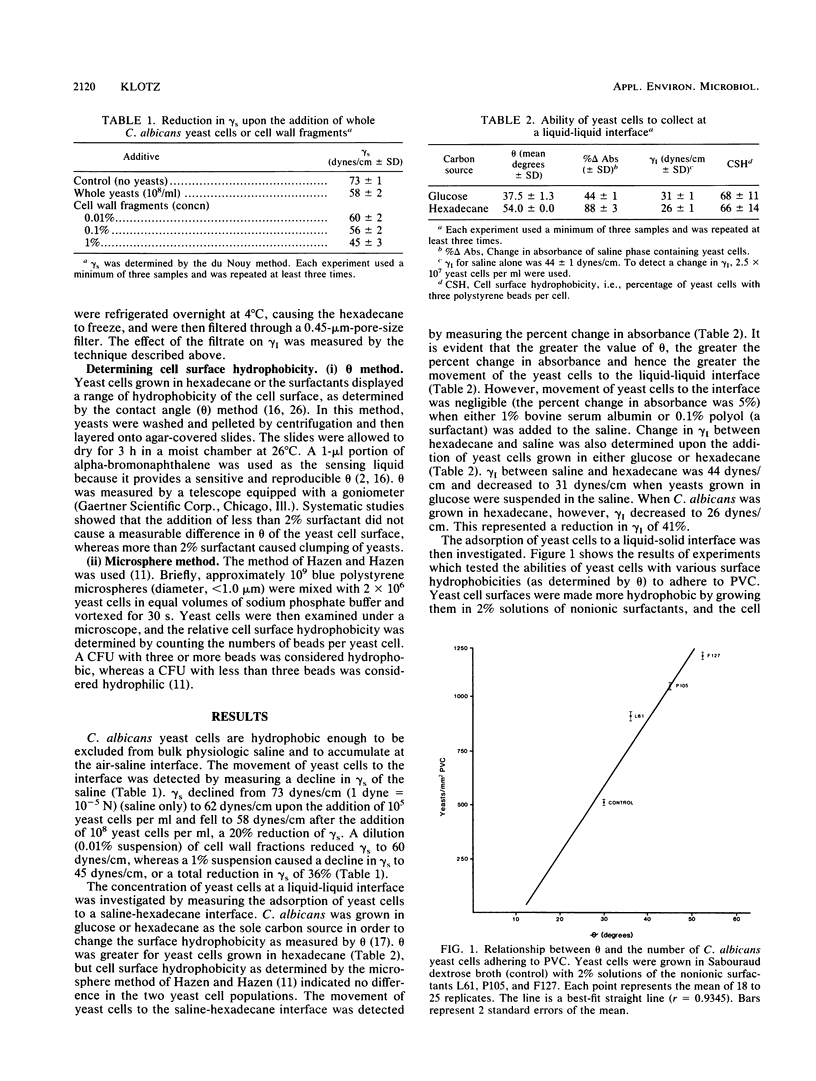
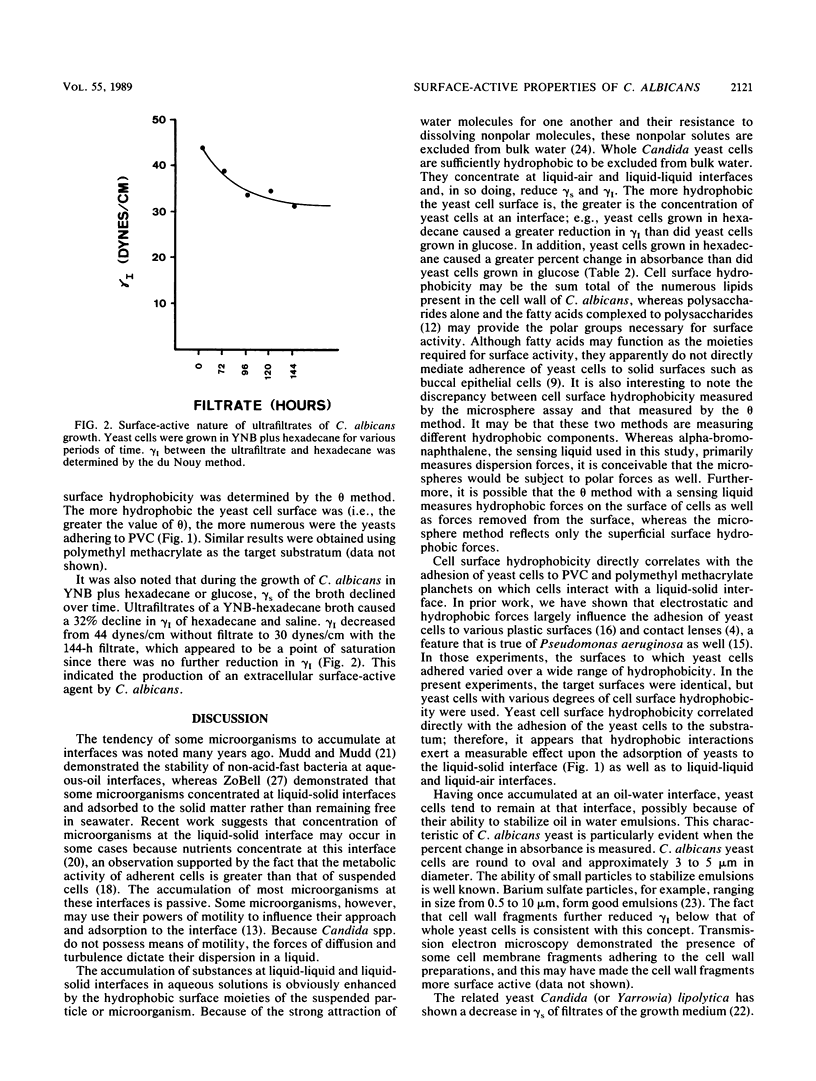
Cell surface hydrophobicity may be an important factor contributing to the virulence of Candida yeast cells. Surface hydrophobic and surface polar groups would be required for a yeast cell to act as a surface-active agent. In this report, the surface activities of whole yeast cells were measured. Yeast cells added at 10(8)/ml reduced the surface tension (gamma s) of saline by 20% as determined by the du Nouy method. A 1% suspension of yeast cell wall fragments reduced gamma s of saline by 36%. Whole yeast cells caused a reduction in interfacial tension (gamma I) between hexadecane and saline. The reduction of gamma I was proportional to the surface hydrophobicity of the yeasts. Yeast cells grown in glucose as the sole carbon source (thus possessing a relatively more hydrophilic cell surface) reduced gamma I by 30%, whereas yeast cells grown in hexadecane (thus possessing a more hydrophobic cell surface) reduced gamma I by 41%. The reduction of gamma I was reversed upon the addition of a strong surfactant. It was also demonstrated that yeast cells blended with nonionic surfactants during growth in a glucose broth in order to change their cell surface hydrophobicity adhered to solid surfaces in direct proportion to their cell surface hydrophobicity. Thus, the surface-active properties of Candida yeast cells may significantly contribute to the accumulation of yeast cells at various biological interfaces such as liquid-solid, liquid-liquid, and liquid-air, leading to their eventual adhesion to solid or tissue surfaces.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antley P. P., Hazen K. C. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect Immun. 1988 Nov;56(11):2884–2890. doi: 10.1128/iai.56.11.2884-2890.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. I., Bloomfield S., Pearce D. B., Tragakis M. Infections with the therapeutic soft lens. Arch Ophthalmol. 1974 Apr;91(4):275–277. doi: 10.1001/archopht.1974.03900060285007. [DOI] [PubMed] [Google Scholar]
- Butrus S. I., Klotz S. A. Blocking Candida adherence to contact lenses. Curr Eye Res. 1986 Oct;5(10):745–750. doi: 10.3109/02713688609000015. [DOI] [PubMed] [Google Scholar]
- Cirigliano M. C., Carman G. M. Isolation of a bioemulsifier from Candida lipolytica. Appl Environ Microbiol. 1984 Oct;48(4):747–750. doi: 10.1128/aem.48.4.747-750.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry C. R., Quie P. G. Fungal septicemia in patients receiving parenteral hyperalimentation. N Engl J Med. 1971 Nov;285(22):1221–1225. doi: 10.1056/NEJM197111252852203. [DOI] [PubMed] [Google Scholar]
- Fischl A. S., Carman G. M. Phosphatidylinositol biosynthesis in Saccharomyces cerevisiae: purification and properties of microsome-associated phosphatidylinositol synthase. J Bacteriol. 1983 Apr;154(1):304–311. doi: 10.1128/jb.154.1.304-311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. F., Chew W. H., Shadomy S., Duma R. J., Mayhall C. G., House W. C. Urinary tract infections due to Candida albicans. Rev Infect Dis. 1982 Nov-Dec;4(6):1107–1118. doi: 10.1093/clinids/4.6.1107. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A., Burns G. R., Elteen K. A., Radwan S. S. Experimental evidence for the role of lipids in adherence of Candida spp. to human buccal epithelial cells. Infect Immun. 1986 Oct;54(1):189–193. doi: 10.1128/iai.54.1.189-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Russell E., Jr, Remington J. S. The compromised host and infection. II. Deep fungal infection. J Infect Dis. 1969 Aug;120(2):169–191. doi: 10.1093/infdis/120.2.169. [DOI] [PubMed] [Google Scholar]
- Kefford B., Marshall K. C. Adhesion of Leptospira at a solid-liquid interface: a model. Arch Microbiol. 1984 May;138(1):84–88. doi: 10.1007/BF00425413. [DOI] [PubMed] [Google Scholar]
- Klotz S. A. A bioemulsifier produced by Candida albicans enhances yeast adherence to intestinal cells. J Infect Dis. 1988 Sep;158(3):636–639. doi: 10.1093/infdis/158.3.636. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Butrus S. I., Misra R. P., Osato M. S. The contribution of bacterial surface hydrophobicity to the process of adherence of Pseudomonas aeruginosa to hydrophilic contact lenses. Curr Eye Res. 1989 Feb;8(2):195–202. doi: 10.3109/02713688908995192. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Zajic J. E. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985 Oct;50(1):97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käppeli O., Fiechter A. Component from the cell surface of the hydrocarbon-utilizing yeast Candida tropicalis with possible relation to hydrocarbon transport. J Bacteriol. 1977 Sep;131(3):917–921. doi: 10.1128/jb.131.3.917-921.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macura A. B. Hydrophobicity of Candida albicans related to their adherence to mucosal epithelial cells. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):491–496. doi: 10.1016/s0176-6724(87)80231-6. [DOI] [PubMed] [Google Scholar]
- Nakahara T., Erickson L. E., Gutierrez J. R. Characteristics of hydrocarbon uptake in cultures with two liquid phases. Biotechnol Bioeng. 1977 Jan;19(1):9–25. doi: 10.1002/bit.260190103. [DOI] [PubMed] [Google Scholar]
- Torres-Rojas J. R., Stratton C. W., Sanders C. V., Horsman T. A., Hawley H. B., Dascomb H. E., Vial L. J., Jr Candidal suppurative peripheral thrombophlebitis. Ann Intern Med. 1982 Apr;96(4):431–435. doi: 10.7326/0003-4819-96-4-431. [DOI] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]


