Abstract
Progressive pulmonary disease and infections with Pseudomonas aeruginosa remain an intractable problem in cystic fibrosis (CF). At the cellular level, CF is characterized by organellar hyperacidification, which results in altered protein and lipid glycosylation. Altered pH of the trans-Golgi network (TGN) may further disrupt the protein processing and packaging that occurs in this organelle. Here we measured activity of the major TGN endoprotease furin and demonstrated a marked upregulation in human CF cells. Increased furin activity was linked to elevated production in CF of the immunosuppressive and tissue remodeling cytokine TGF-β and its downstream effects, including macrophage deactivation and augmented collagen secretion by epithelial cells. As furin is responsible for the proteolytic processing of a range of endogenous and exogenous substrates including growth factors and bacterial toxins, we determined that elevated furin-dependent activation of exotoxin A caused increased cell death in CF respiratory epithelial cells compared with genetically matched CF transmembrane conductance regulator–corrected cells. Thus elevated furin levels in CF respiratory epithelial cells contributes to bacterial toxin–induced cell death, fibrosis, and local immunosuppression. These data suggest that the use of furin inhibitors may represent a strategy for pharmacotherapy in CF.
Introduction
Cystic fibrosis (CF), the most common lethal inheritable disease in Caucasians (1), is caused by mutations in the gene encoding a chloride channel termed CF transmembrane conductance regulator (CFTR), resulting in multi-organ dysfunction. The main life-shortening pathology in CF is associated with chronic Pseudomonas aeruginosa lung infections (1). Mutations in CFTR have pleiotropic effects on the function of other ion transporters (2, 3). Of particular importance is the CFTR-dependent regulation of the amiloride-sensitive epithelial sodium channel in respiratory epithelial cells (2, 3), causing electrolyte and fluid hyperabsorption in the CF lung (2, 4) and hyperacidification of intracellular organelles of CF lung epithelial cells including trans-Golgi network (TGN) and endosomal compartments (5–10). In addition, there is an abundance of proposed defects related to pathology in CF, including defective phagosomal acidification in macrophages (11), altered P. aeruginosa uptake by respiratory epithelial cells (12), impaired glycosylation (13), enhanced Toll-like receptor activation (14), reduced levels of iNOS (15), and disrupted cholesterol transport (16). The wide range of deficiencies in CF is a reflection of the multifactorial nature of problems in CF, and only seldom can multiple manifestations of lung disease be ordered in a sequence of causal relationships. One exception is the following series of linked abnormalities (5): (a) CFTR loss–associated aberrant sodium transport; (b) organellar hyperacidification due to uninhibited sodium transport out of the organellar lumen, thus permitting higher proton accumulation; (c) altered protein and lipid glycosylation due to TGN hyperacidification; (d) increased bacterial adhesion due to altered glycosylation of cell surface–destined macromolecules; and (e) elevated inflammation in response to bacterial products due to hyperacidified endosomes in which many Toll-like receptors function.
The reports on altered products of glycosylase action in the TGN of CF respiratory epithelial cells (6, 13) indicate that the hyperacidified lumen of this organelle (6) may have other consequences on the properties of CF cells. In addition to being the organelle carrying out terminal glycosylation modifications of proteins destined for secretion or for sorting to the plasma membrane, TGN is a biosynthetic station in which a number of proteins are processed from their pro forms to mature proteins, with the endoprotease furin being a main proprotein convertase in this compartment (17). Furin is primarily located in the TGN (17), but it also readily traffics to the plasma membrane and recycles via the endosomal organelles. The dynamic distribution of furin enables it to cleave and activate numerous intracellular and extracellular proproteins in both the biosynthetic and endocytic pathways (17). Furin is involved in the processing of the substrates, containing the minimal basic amino acid RXXR recognition motif, such as coagulation factors, hormones and growth factors, cell-surface receptors, and extracellular matrix proteins (17). However, furin is not limited to processing of endogenous cellular proteins; it can be co-opted by bacterial toxins and viral coat proteins for maturation and activation in the host (17). We wondered, given the TGN dysfunction, as evidenced by defective sialylation of CF proteins and glycolipids (6, 13), whether furin activity was perturbed in CF respiratory epithelial cells and what would be the physiological consequences of potentially altered furin action. We report here that CF cells show increased furin activity, which explains the abnormally high TGF-β levels in CF cells (18), since pro–TGF-β is one of the furin substrates. Moreover, we demonstrate that elevated furin levels in CF cells renders them more sensitive to the main P. aeruginosa toxin (19), exotoxin A (ExoA), which requires furin-dependent processing for activation.
Results
Increased furin activity in CF cells.
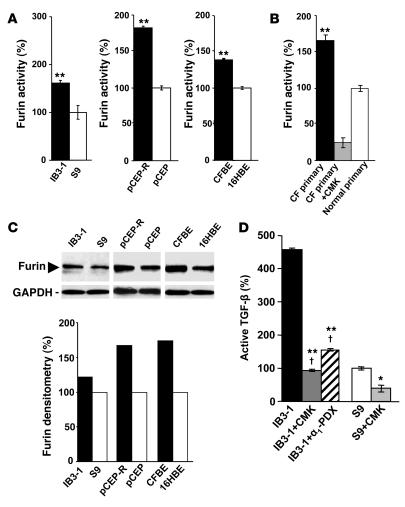
To determine furin activity, permeabilized cells were assayed with the fluorogenic peptide substrate boc-RVRR-amc, which is cleaved to liberate a fluorescent product, reflecting furin activity. A CF bronchial epithelial cell line, IB3-1, from a patient with the DF508/W1282X CFTR mutant genotype (20), displayed a higher furin activity than the CFTR-corrected, genetically matched S9 cells (20) (Figure 1A). Increased furin activity was also found when other CF and normal cells were compared, including pCEP-R cells, in which the CF phenotype is induced using a dominant-negative construct expressing the R domain of CFTR (Figure 1A). Furin activity was also examined in primary human lung epithelial cells (Figure 1B). The assay for furin was controlled by including the furin inhibitor decanoyl-RVKR-chloromethylketone (CMK) in the reaction mixture (Figure 1B). Higher levels of furin in CF cells were detected by western blots (Figure 1C). Thus CF cells have elevated levels of furin, and this translates into a higher furin activity in CF cells compared with normal cells.
Figure 1. Furin levels are elevated in CF cells.
Furin activity was measured by monitoring cleavage of fluorogenic substrate boc-RVRR-amc in extracts from lung epithelial cells. One unit of activity was defined as the amount of enzyme required to liberate 1 pmol of AMC from boc-RVRR-amc. (A) IB3-1, pCEP-R, and CFBE (all 3 cell lines with a CF phenotype) and S9, pCEP, and 16HBE (all 3 cell lines with CFTR-corrected or non-CF phenotype). (B) Furin activity in primary human (CF and normal) lung epithelial cells measured in the presence or absence of the furin inhibitor CMK. (C) Furin western blot and densitometry analysis. (D) Human lung epithelial cells after treatment with furin inhibitors CMK and α1-PDX. Production of TGF-β was measured in IB3-1 (CF) and S9 (CFTR corrected) epithelial cells using TGF-β–responsive luciferase assay. CMK, 50 μM; α1-PDX, 5 μM. Data are presented as percentage of furin or TGF-β, for which 100% corresponds to protein levels in non-CF cells (n = 3 experiments). Mean ± SEM (*P < 0.05, **P < 0.01; †P ≥ 0.05).
Increased furin activity is responsible for elevated TGF-β production by CF cells.
CF cells produce more TGF-β than do normal cells (18) (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI31499DS1). We tested whether elevated levels of TGF-β are due to increased activity of furin in CF cells. Pro–TGF-β is processed by furin in the TGN, an organelle that is hyperacidified in CF (6, 9), releasing the mature TGF-β. We tested whether excess TGF-β is due to altered furin activity in CF cells by examining the effects of furin inhibitors CMK and Portland α1–antitrypsin (α1-PDX). IB3-1 cells were incubated in media with 50 μM CMK (directly tested for inhibitory action on furin; Figure 1D) or 5 μM α1-PDX for 24 hours, and bioactive TGF-β was determined using PAI/L assay detecting biologically active TGF-β. The amounts of TGF-β released by IB3-1 cells were reduced by CMK and α1-PDX treatment (P < 0.01) (Figure 1D). Furin inhibitors brought the levels of TGF-β in IB3-1 cells to those in genetically matched, CFTR-corrected cells (S9) (P < 0.05). These results show that increased furin activity in CF epithelial cells governs the abnormally high TGF-β levels and that this can be corrected with furin inhibitors.
Normalization of furin activity in CF cells.
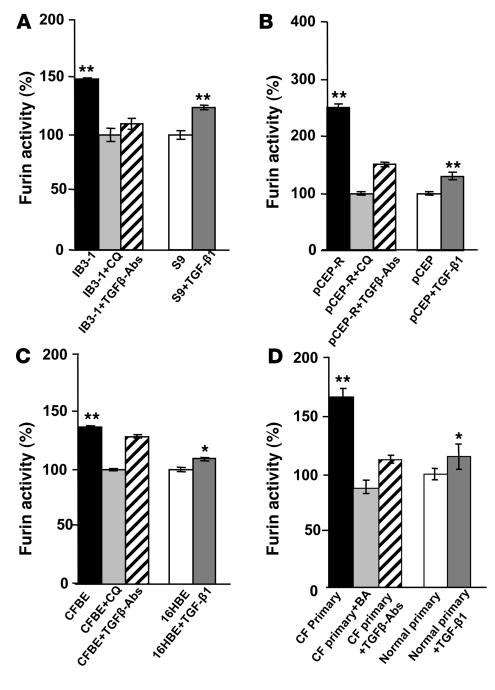
Furin is proteolytically processed into its mature form within the TGN (17) as a step necessary for its convertase action. Chloroquine corrects the hyperacidification of a subset of intracellular organelles that are abnormally acidic in CF lung epithelial cells (5, 6). Chloroquine restored the pH of the TGN in IB3-1 mutant cells to levels matching those in CFTR-transfected S9 cells (Supplemental Figure 2). We tested whether furin processing is enhanced in CF lung epithelial cells due to the hyperacidification of the TGN. When CF cells were incubated with 0.1 mM chloroquine, total furin activity was significantly inhibited in all cells tested (Figure 2, A–C). The effect of hyperacidification was additionally confirmed by using primary human CF lung epithelial cells and applying another acidification antagonist, bafilomycin A1, an inhibitor of vacuolar H+ ATPase responsible for acidification of intracellular organelles including endosomes and TGN. Bafilomycin A1 brought furin activity levels in CF primary cells to levels seen in normal primary cells (Figure 2D). These results indicate that excessive furin processing/activation in CF cells can be normalized by correcting organellar pH with chloroquine.
Figure 2. Chloroquine and blocking antibodies against TGF-β normalize furin activity in CF cells.
Cells were incubated in full medium with 10 ng/ml TGF-β1 or in the presence of 5 μg/ml TGF-β antibodies or were treated with 0.1 mM chloroquine (CQ). (A) IB3-1 (CF) and S9 (CFTR corrected) cells. (B) pCEP-R (overexpressing the CFTR R domain) and pCEP (mock-transfected) cells. (C) CFBE (CF) and 16HBE (normal) cell lines. (D) Additionally, furin levels were normalized with 100 nM bafilomycin A1 (BA) or 50 μM furin inhibitor (CMK) in primary (CF and normal) human lung epithelial cells. Data are presented as percentage of furin, where 100% corresponds to furin activity in non-CF cells (n = 3 experiments). Mean ± SEM (*P < 0.05, **P < 0.01).
Higher furin levels in CF cells and positive feedback loop with TGF-β.
TGF-β is known to induce the expression of furin in a positive feedback loop (21). To test whether these relationships contribute to elevated furin levels in CF, we treated CF cells with blocking antibodies against TGF-β and measured furin (Figure 2, A–D). After treatment with blocking antibodies against TGF-β, furin activity decreased in CF cells. Irrelevant antibodies had no effect (data not shown). In a converse experiment, pretreatment of normal cells (S9, pCEP, 16HBE, and primary normal human lung epithelial cells) with TGF-β1 caused elevated furin activity (Figure 2, A–D). Thus TGF-β and furin are linked in a positive amplification loop in respiratory epithelial cells, which augments furin levels in CF.
Elevated furin and TGF-β levels increase collagen production.
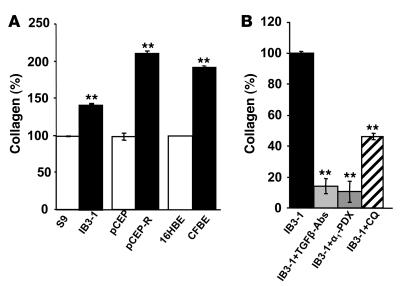
In pulmonary fibrosis, respiratory epithelial cells produce more collagen with TGF-β being the most direct stimulator of collagen production (22, 23). To examine whether increased TGF-β levels lead to altered collagen production by CF cells, collagen levels were measured using Sircol collagen assay (Figure 3A). Total soluble collagen was increased in all CF lines tested (IB3-1, pCEP-R, and CFBE) relative to normal or CFTR-corrected respiratory epithelial cells. Blocking TGF-β antibodies decreased the soluble collagen content in IB3-1 cells. A furin inhibitor, α1-PDX also downregulated the level of soluble collagen (Figure 3B). The total soluble collagen secretion was markedly decreased when IB3-1 cells were treated with chloroquine (Figure 3B).
Figure 3. TGF-β contributes to increased collagen production.
(A) Total soluble collagen (%) was measured in the media from human airway epithelial cells after 24 hours. Media containing collagen from non-CF (open bars) and CF (filled bars) cell lines. (B) IB3-1 cells incubated in full medium with 5 μg/ml blocking TGF-β antibodies in the presence of furin inhibitor (α1-PDX) or treated with 0.1 mM chloroquine. 100% corresponds to collagen levels in non-CF cells. Mean ± SEM (n = 3 experiments; **P < 0.01).
TGF-β suppresses antipseudomonal action of macrophages and diminishes iNOS expression in CF epithelial cells.
TGF-β contributes to the persistence of certain infections by downregulating the antimicrobial activity of macrophages (24–26). To determine whether the increased TGF-β affected P. aeruginosa killing by macrophages, we examined the effect of TGF-β on the ability of primary human peripheral blood monocyte-derived macrophages (MDM) to eliminate bacteria (Supplemental Figure 3). The TGF-β–treated MDM displayed a 2-fold reduction in their ability to kill P. aeruginosa (Supplemental Figure 3A). To test directly whether increased TGF-β produced by CF respiratory epithelial cells can affect the ability of human macrophages to kill P. aeruginosa, we conducted coculture experiments with CF primary cells and MDM. When cocultures consisting of CF primary lung epithelial cells and MDM were infected with P. aeruginosa, bacterial survival decreased when blocking TGF-β antibodies were added (Supplemental Figure 3B). A similar enhancement in bacterial killing was observed when a furin inhibitor (CMK) was used to pretreat respiratory epithelial cells (Supplemental Figure 3B). TGF-β blocking antibody and CMK enhanced the killing ability of macrophages cocultured with CF respiratory epithelial cells to equal that of MDM coculture with normal respiratory epithelial cells. These observations show that TGF-β inhibits P. aeruginosa elimination by human macrophages and that TGF-β production by respiratory epithelial cells affects the ability of macrophages to kill this bacterium. We also found that altered TGF-β contributes to lower than normal expression of iNOS (which too plays an antibacterial role) in CF lung epithelial cells (Supplemental Figure 3, C–E). These results link increased furin activity and elevated TGF-β levels to defective antipseudomonal capacity of the CF lung.
CF cells are more sensitive to P. aeruginosa ExoA.
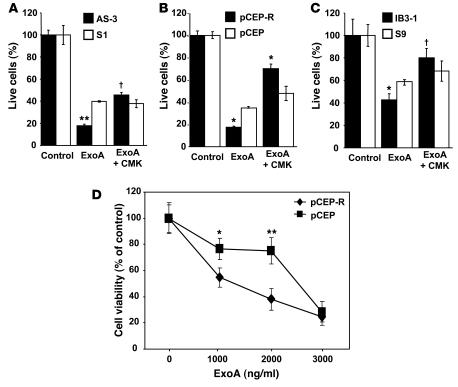
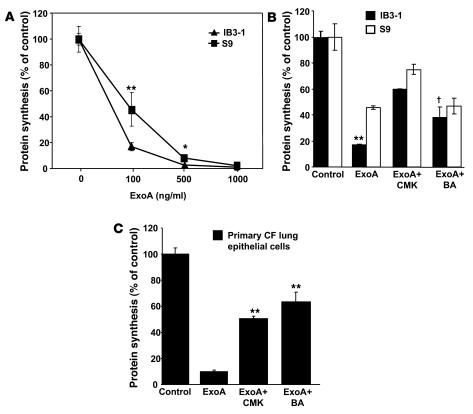
ExoA is detectable in sputum samples of patients infected with P. aeruginosa (27, 28). ExoA is processed by furin to produce a C-terminal 37-kDa fragment which translocates to the cytosol and inhibits protein synthesis (29–31). ExoA is cleaved by furin in acidic intracellular compartments to produce an active toxin that ADP-ribosylates elongation factor 2, leading to cessation of protein synthesis, blocking of Sec61 translocon, and cell death (32–35). The CF and normal cell pairs were treated with ExoA for 24 hours (Figure 4, A–C), and effects on cell viability determined by WST-1 cytotoxicity assay. In Figure 4, A–C, the effects of ExoA-induced cytotoxicity were significantly higher in CF cell lines. Inhibition of furin with CMK resulted in protection from the toxin and increase in cell viability (Figure 4, A–C). Results from an experiment in which pCEP-R cells and their non-CF counterpart pCEP cells were treated with increasing concentrations (0 to 3,000 ng/ml) of ExoA are displayed in Figure 4D and indicate that CF cells showed increased sensitivity to ExoA over a range of toxin concentrations.
Figure 4. CF cells are more sensitive to P. aeruginosa ExoA.
CF and matched non-CF respiratory epithelial cells were cultured overnight in 96-well plates with media containing 10% serum. Cells were incubated with ExoA for 24 hours. Cell viability was measured by adding WST-1 as described in Methods. (A) AS1 normal human respiratory epithelial cells stably transfected with CFTR antisense construct (inducing CF phenotype) and their matching control S1 (cells stably transfected with the sense construct). (B) pCEP-R (overexpressing the dominant-negative CFTR R domain) and pCEP (mock-transfected). (C) IB3-1 and S9 (CFTR corrected). Human lung epithelial cells were incubated with furin inhibitor CMK (50 μM) for 24 hours at 37°C. (D) Dose-dependent cytotoxicity of ExoA. Lung epithelial cells were treated for 24 hours with increasing concentrations of P. aeruginosa ExoA (0 to 3,000 ng/ml). Data are presented as percentage of live cells, for which 100% corresponds to ExoA untreated cells (n = 3 experiments). Mean ± SEM (*P < 0.05, **P < 0.01, †P ≥ 0.05).
The hypersusceptibility of CF cells to ExoA was also tested using another, more sensitive assay, based on ExoA inhibition of protein synthesis. The concentration of ExoA had to be titrated down to 100 ng/ml to observe differences between IB3-1 and S9 cells after 24 hours of incubation with the toxin (Figure 5A). The primary cells showed even higher sensitivity to ExoA in the protein synthesis inhibition assay, and to observe the differences between primary CF and normal lung epithelial cells, the incubation with the toxin was shortened to 4 hours (Supplemental Figure 5A). The difference between CF (IB3-1) and CFTR-corrected (S9) cells was abrogated by the inclusion of bafilomycin A1 and was significantly diminished with the furin inhibitor CMK (Figure 5B). The primary CF human lung epithelial cells showed similar response to CMK or bafilomycin A1 inhibitors (Figure 5C and Supplemental Figure 5). These results demonstrate that CF cells are more sensitive to ExoA than are CFTR-corrected or non-CF cells.
Figure 5. CF cells show increased sensitivity to ExoA inhibition of protein synthesis.
CF and non-CF cells were incubated in media containing either increasing concentrations of ExoA for 24 hours (A) or 100 ng ExoA/ml (B) with IB3-1 and S9 (CFTR-corrected) cells. (C) CF primary lung epithelial cells in the presence or absence of furin inhibitor CMK (50 μM) or treated with 100 nM bafilomycin A1 (BA). Protein synthesis levels were determined by measuring the incorporation of [3H] leucine into TCA-precipitable cellular proteins and are expressed as percentage of control cells that received no toxin. Mean ± SEM for 3 separate experiments; *P < 0.05, **P < 0.01, †P ≥ 0.05.
Increased furin activity causes ExoA-induced cytotoxicity in CF.
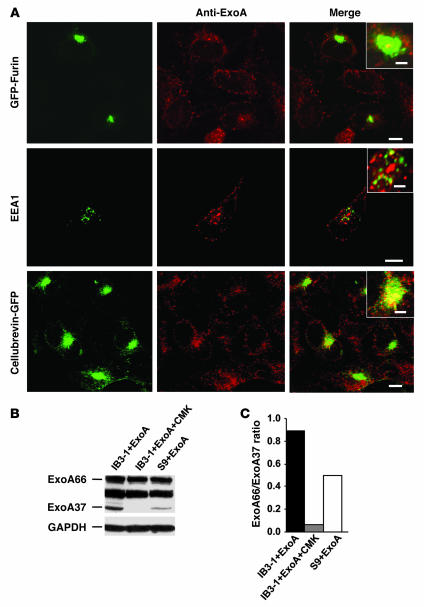
ExoA was distributed in respiratory epithelial cells, in keeping with its retrograde trafficking route (Figure 6A). Only a limited direct overlap or juxtaposition could be seen between GFP-furin and ExoA (Figure 6A). Instead, the strongest localization of ExoA was with cellubrevin-pHluorin GFP (Figure 6A), which localizes to the recycling endosome, also hyperacidified in CF cells (8, 9). The bulk of cellular furin under steady-state conditions was, as expected, in the Golgi/TGN region, with no discernible differences between CF and CFTR-corrected cells (Supplemental Figure 4).
Figure 6. Furin-dependent processing of ExoA is increased in CF cells.
(A) Localization of ExoA in CFTR mutant IB3-1 cells by fluorescence microscopy (insets, close apposition). Cells were transfected with GFP-furin or cellubrevin-pHluorin GFP and incubated with ExoA conjugated to secondary Alexa Fluor 568 (red). EEA1 was visualized using primary human anti-EEA1 antibody and Alexa Fluor 468–conjugated antibody (green). Scale bars: 5 μm; 2 μm (insets). (B) SDS-PAGE analysis of ExoA processing in IB3-1 (CFTR mutant) cells, IB3-1 cells incubated with CMK (50 μM), and S9 (CFTR-corrected IB3-1) cells. Proteins in cell extracts were separated by SDS-PAGE and immunoblotted with anti-ExoA. ExoA66, full size, inactive ExoA toxin precursor; ExoA37, proteolytically processed 37-kDa active ExoA fragment. (C) Densitometry analysis of ExoA66/ExoA37 ratio.
Nevertheless, it is known that ExoA and furin transit through the same intracellular organelles (29, 31, 36), and either increased furin quantity or its activity under more acidic conditions in CF cells may explain increased cytotoxicity of ExoA to CF cells. Furin processes ExoA to generate a 37-kDa C-terminal fragment that translocates to the cytosol and ADP-ribosylates elongation factor 2, thereby causing cell death (29, 31, 36). To test whether CF cells displayed increased ExoA processing capacity and conversion into its active 37-kDa form, IB3-1 (CF) or S9 (CFTR-corrected) cells were exposed to ExoA (1,000 ng/ml) for 24 hours at 37°C. Products were analyzed by SDS-PAGE followed by immunoblotting and probed with the ExoA polyclonal antibody, which reacts with full-size ExoA (66 kDa) and the active proteolytic fragment of 37-kDa ExoA. Cleavage of ExoA was enhanced in IB3-1 cells relative to S9 cells, generating more of the 37-kDa fragment (Figure 6, B and C). No 37-kDa product was seen when ExoA was incubated with CMK furin inhibitor (Figure 6, B and C), showing that generation of the active toxin depended on furin.
Discussion
The protein convertase furin quantity and activity are elevated in CF respiratory epithelial cells and lead to a number of previously unappreciated physiological consequences stemming from what we believe to be a newly recognized aberration in CF. The downstream consequences include increased levels of immunosuppressant and tissue remodeling cytokine TGF-β (Figure 7), with attendant downregulation of macrophage bactericidal functions, lowered iNOS expression in CF epithelial cells, and elevated secretion of collagen; and increased capacity of CF respiratory epithelial cells to activate the main toxic product of P. aeruginosa, ExoA, rendering them more susceptible to the effects of this toxin as measured by cell death (Figure 7). Of note is that these newly uncovered deficiencies are linked to the previously reported organellar hyperacidification (TGN and endosome) in CF, thus affirming previous findings (6, 9, 18). Furthermore, our observations are germane to the known but, to our knowledge, hitherto unexplained pathology in CF and thus clarify issues in CF. The newly recognized spectrum of abnormalities in CF cells and tissues does come with a silver lining, since all newly described defects are interconnected and can be treated at their root by normalizing organellar pH or by inhibiting furin.
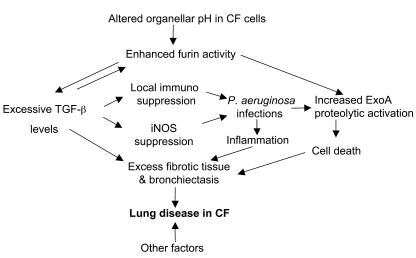
Figure 7. Model depicting pathological cascade in CF based on increased furin activity and elevated TGF-β levels.
Low intra-organellar (TGN and recycling endosome) pH in CF cells increases furin activity in respiratory epithelial cells, which causes a cascade of downstream effects. Left: Increased TGF-β maturation due to its enhanced processing contributes to suppression of iNOS and increased secretion of fibrotic tissue components (collagen). Increased TGF-β further augments furin levels in a positive feedback loop. Increased TGF-β leads to tissue fibrosis contributing to bronchiectasis, and local immunosuppression. Right: Increased activation of P. aeruginosa ExoA by furin in the TGN leads to higher cytotoxicity. These defects underlie the majority of salient aspects of lung disease in CF.
Furin, a ubiquitously expressed proteolytic-processing convertase, has been previously implicated, although not in CF, in a number of pathological processes (17). The increased furin levels and activity in CF can explain the recently reported elevated TGF-β in CF cells (18). Pro–TGF-β is normally processed by furin (17) releasing the mature TGF-β. Although additional factors may contribute to the higher active TGF-β levels in CF, furin processing of TGF-β probably plays an important role, since it showed a rate-limiting effect even in non-CF cells, based on decreased TGF-β levels in the presence of furin inhibitors (Figure 1D). The hyperacidification of TGN in CF most likely initiates increased TGF-β processing by furin. A positive feedback loop between TGF-β and furin (21), also observed in our work, amplifies levels of both furin and TGF-β in CF, leading to increased furin amounts detectable by immunoblots. The bulk of cellular furin is located in TGN (17) but it traffics appreciably to and from the plasma membrane, passing through endosomal organelles that are also hyperacidified in CF respiratory epithelial cells (7–9). In addition to its intracellular action, furin is released from cells to process a number of extracellular substrates (17). Excess furin released from CF cells could play a role in the final activation of secreted TGF-β by liberating it from latent complexes where it is trapped by association with other proteins, through a previously described process distinct from the intracellular processing of the TGF-β pro form (37). TGF-β activation may also be exacted within the hyperacidified TGN in CF cells, since acidification is the most efficient way of dissociating TGF-β from other components within the latent TGF-β complexes (38).
Our measurements show that CF respiratory epithelial cells secrete more collagen than their CFTR-corrected counterparts. TGF-β is one of the most potent regulators of connective tissue synthesis and controls deposition of profibrotic extracellular matrix protein. It plays a role in lung tissue remodeling following injury (39), and despite its crucial role in tissue repair, a fine balance is required, as increased levels of TGF-β or its receptors have been shown to result in severe pulmonary fibrosis in rat models (40). A genetic comprehensive study has indicated that TGF-β gene modifies lung disease severity in CF (41). Thus elevated TGF-β activity downstream of furin upregulation may explain salient aspects of the failure of tissue to remodel appropriately following injury in CF, including bronchiectasis and fibrosis. As shown here, chloroquine, furin inhibitors, or TGF-β blocking antibodies normalize collagen production, confirming the cascade depicted in Figure 7. TGF-β has been detected in CF specimens, including histological preparations (42) and bronchoalveolar lavage fluid (BALF) (43). A recent direct measurement of TGF-β1 in BALF from 43 CF patients (43) found a correlation between TGF-β1 in BALF and reticular basement membrane thickness (RBMT) in endobronchial biopsies in CF. Since the same study reported an increase in RBMT in CF compared with normal subjects, at least 1 parameter of CF respiratory disease shows a positive correlation with TGF-β levels in BALF in CF. In further agreement with our observations, the study by Hilliard et al. (43) also showed a 5-fold increase in collagen levels in BALF from CF compared with normal subjects, which can be explained by elevated TGF-β1 reported here. Lastly, CF patients with the TGF-β1 high-producer genotype have more rapid deterioration in lung function (44, 45), in keeping with our findings and with the results of studies in human populations showing that TGF-β is a genetic modifier in CF (41).
TGF-β is a recognized modulator of immune function and can act as an immunosuppressant. TGF-β plays a central role in the pathogenesis of several chronic infectious diseases; a range of macrophage-deactivating properties that lead to inhibition of macrophage ability to kill several pathogens, including Trypanosoma cruzii, Mycobacterium tuberculosis, and Leishmania major, has been ascribed to TGF-β (24–26). Although macrophage function in CF has not been extensively studied, a recent report with CF transgenic mice has indicated a potential problem in the ability of macrophages from CF animals to kill P. aeruginosa (11). The evidence presented here supports the possibility that increased TGF-β production by respiratory epithelial cells affects the ability of phagocytes to control P. aeruginosa in CF. A local suppression of macrophage function by TGF-β derived from respiratory epithelial cells is compatible with the lung-restricted immunodeficiency in lieu of compromised systemic immunity in CF, a feature of CF that has previously eluded satisfactory explanation.
In addition to the above trans effects, an autocrine action of elevated TGF-β observed here, most likely along with the previously reported STAT1 mechanisms (46), may lead to the lower than normal iNOS expression in CF respiratory epithelia (15, 47). This is compatible with TGF-β acting as a known suppressor of iNOS expression (48). Since iNOS-generated nitric oxide has antibacterial actions in addition to its other physiologic functions, iNOS suppression by TGF-β may further compound the ability of the CF lung to combat bacterial pathogens.
The most surprising aspect of our study is the finding that P. aeruginosa toxin ExoA causes significantly higher cell death in CF cells relative to CFTR-corrected controls. This is caused by the cryptic ability of CF cells to activate ExoA more efficiently than normal cells. Furin-dependent cleavage of ExoA occurs at low pH and takes place in the TGN and endosomes, where the acidic environment induces a conformational change in ExoA that renders it furin susceptible (30, 49). The increased processing of ExoA in CF is most likely the result of a combination of elevated furin levels and lower than normal pH in the relevant organelles of the CF respiratory epithelial cells. In order for ExoA to exert its cytotoxic activity, it has to be proteolytically converted to produce the 37-kDa lethal fragment, which translocates to the cytosol and inhibits protein synthesis (30, 31, 49). As shown here, inhibition of furin can block proteolytic activation of ExoA and exotoxin-mediated death of human respiratory epithelial cells. ExoA is a P. aeruginosa product expressed in the majority of CF patients, and ExoA serology can be used, with various levels of sensitivity, to monitor onset of P. aeruginosa colonization, chronic infection, progress of antimicrobial therapy, or recrudescence (50). Thus it is likely that the hypersusceptibility of CF epithelial cells to this toxin produced by P. aeruginosa (Supplemental Figure 5B) plays a role in CF pathogenesis.
Our work demonstrates that the serine protease furin is generated in excess by CF respiratory epithelial cells. The following model emerges: increased furin action brings about elevated production of fibrotic tissue constituents via TGF-β (Figure 7). Furthermore, the cytotoxicity of ExoA produced by P. aeruginosa, the most prevalent CF pathogen, is increased due to enhanced ExoA processing via furin in CF cells. We propose that these 2 effects synergize, contributing to irreversible tissue damage, fibrosis, and loss of elasticity, thus playing a substantial role in bronchiectasis and resulting in irreversible pathology in the CF lung, which leads to respiratory failure and premature death. Based on the reports that the organellar pH defect in CF can be normalized using the FDA-approved drug chloroquine (6, 9, 18), we applied this compound here to show correction of the increased furin levels in CF. Chloroquine is widely used for treatment of chronic inflammatory diseases such as rheumatoid arthritis and is being tested in clinical studies for its effects in CF (http://www.cff.org/research/ClinicalResearch/Find/?IDS=1&SearchAll=1?). In addition to organellar hyperacidification targeted by chloroquine, the beneficial effects of furin inhibitors described here strongly suggest that furin is a suitable pharmacological target and that provides new opportunities for therapeutic intervention in correcting a set of linked abnormalities in CF.
Methods
Cells and constructs.
IB3-1 is a human bronchial epithelial cell line derived from a CF patient with a ΔF508/W1282X CFTR mutant genotype (51). S9 is an IB3-1 derivative stably transfected with a functional CFTR and is corrected for chloride conductance (20). 9HTEo- cell derivatives stably overexpress the dominant-negative CFTR R domain (pCEP-R) or are mock-transfected (pCEP), acting as normal lung epithelial cells (52). NuLi-1 (normal lung) cell line was derived from human airway epithelium of normal CFTR genotype; CuFi-3 (CF) cell line was derived from a CF patient (53). The simian virus 40 large T antigen–transformed human bronchial epithelial cell line 16HBE14o-, which maintains tight junctions (54, 55), and CF human bronchial epithelial cells CFBE41o- (CFBE, homozygous for the ΔF508 mutation) were from D. Gruenert (California Pacific Medical Center Research Institute, San Francisco, California, USA). The 16HBE cells were transfected with plasmids expressing the first 131 nucleotides of cftr in either the sense (S1) or antisense (AS3) orientations (S6). CF primary cells CFB-6-04 were from J. Zabner (University of Iowa, Iowa City, Iowa, USA) and human bronchial epithelial cells (NHBE) from Lonza Inc. Mink lung epithelial cells transfected with the plasminogen activator inhibitor-1/luciferase (PAI/L) DNA construct were from D.B. Rifkin (New York University School of Medicine, New York, New York, USA). Human peripheral blood monocyte-derived macrophages were isolated from individual donors by countercurrent centrifugal elutriation, and adherent monocytes were cultured in RPMI 1640 medium supplemented with 10% FBS (HyClone). All cells were grown at 37°C in 5% CO2 under standard conditions. GFP-furin was from G. Thomas (Vollum Institute, Portland, Oregon, USA). Cellubrevin-pHluorin GFP was from J. Rothman (Memorial Sloan-Kettering Cancer Center, New York, New York, USA).
Furin enzyme activity assay.
Adherent cells (1.5 × 104) were assayed in the presence of 0.25% Triton X-100 as a permeabilizing agent using boc-RVRR-amc (100 μM) (Bachem) as a furin substrate. Fluorescence was measured at 380-nm excitation and 460-nm emission wavelengths (37, 57). The data were normalized to cell density by parallel staining of another 96-well plate with crystal violet. For released furin activity, supernatants were cleared of cell debris by centrifugation and assayed as described above. Data were normalized to the protein concentration in the supernatant.
PAI/L bioassay for TGF-β.
The PAI/L assay using mink lung epithelial cells (MLEC) stably transfected with a luciferase reporter construct under the control of a TGF-β–specific, truncated PAI-1 promoter was performed as described previously (58). Total TGF-β was determined following acid activation of latent TGF-β in conditioned medium from respiratory cell cultures by incubation of samples with 4 N HCL for 1 hour, followed by neutralization with 4 N NaOH prior to assaying. Luciferase activity in conditioned medium was determined using an Orion Microplate Luminometer (Berthold Detection Systems). The amounts of TGF-β were normalized per cell number.
Western blot analysis.
Protein samples were prepared by homogenizing lung epithelial cells in lysis buffer on ice. Protein concentrations were determined using a micro-BCA protein assay reagent kit (Pierce Biotechnology). Separated proteins were transferred onto nitrocellulose membranes, blocked, and probed overnight with antibody against furin (MON-152; Alexis Biochemicals), rabbit anti-ExoA (Sigma-Aldrich), and rabbit anti-iNOS antibody (Transduction Laboratories). Immune complexes were detected with horseradish peroxidase-conjugated IgG (Pierce Biotechnology). Signal was visualized by incubating with Super Signal chemiluminescent substrate (Pierce Biotechnology). Densitometry analysis of western blots was performed using NIH ImageJ 1.62 software.
Collagen measurements.
Total soluble collagen was measured in culture supernatants by the Sircol assay (Biocolor).
Cytotoxicity assay.
Airway epithelial cells (105/ml) were seeded to 96-well plates 1 day before the assay. ExoA (Calbiochem) at 1,000 ng/ml concentration was incubated with the cells for 24 hours at 37κ C. Cell viability was monitored as previously described (59) using the compound WST-1 (4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) (Roche Applied Science). This dye reflected the activity of mitochondrial dehydrogenase present in living cells; the difference in the absorbencies at 450 and 630 nm was measured 2 hours after addition to cells. The inhibitory concentration of CMK (Calbiochem) for ExoA-mediated cell death was assessed, and 50 μM was employed in subsequent experiments.
Protein synthesis inhibition assay.
Incorporation of [3H] leucine into newly synthesized proteins was used to assess the effect of ExoA on CF cells as previously described (60). Cells grown to 50%–80% confluency in 12-well plates were exposed to ExoA (Calbiochem) for 24 hours, bathed with leucine-free medium for 30 minutes, and incubated for 1 hour with 1 μCi [3H] leucine/ml in HEPES-buffered, leucine-free media. The medium and extracellular radiolabel were then removed and replaced with 10% trichloroacetic acid (TCA) in PBS for 1 hour at 4°C. After a second 10-minute wash with 10% TCA-PBS to remove TCA-soluble radiolabel, the cells were solubilized. The numbers of TCA-precipitable cpm were determined by scintillation counting. Data are presented as a percentage of protein synthesis compared with that in cells that were not challenged with toxin. Samples were run in triplicate, and the experiment was repeated at least twice for each cell line.
Fluorescence microscopy.
All microscopy methods are described in Supplemental Methods.
Statistics.
All experiments were performed 3 times. Data are expressed as mean ± SEM. All statistical analyses were carried out using Fisher’s protected least significant difference post hoc test (ANOVA) (SUPERANOVA version 1.11; Abacus Concepts).
Supplementary Material
Acknowledgments
We thank P. Zeitlin for IB3-1 and S9, J. Zabner for primary human CF lung epithelial cells (CFB-6-04), NuLi and CuFi cells, P. Davis for 9HTEo- cells, D. Gruenert for 16HBEo- and CFBE41o- cells, D. Rifkin for MLEC, and R. Elmaoued, S. McGinn, and J. Chua for help with experiments. W. Ornatowski was a Cystic Fibrosis Foundation postdoctoral fellow. This work was supported by NIH grants AI 50825 and AI 31139.
Footnotes
Nonstandard abbreviations used: CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; CMK, decanoyl-RVKR-chloromethylketone; ExoA, exotoxin A; α1-PDX, Portland α1-antitrypsin; TGN, trans-Golgi network.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:3489–3497 (2007). doi:10.1172/JCI31499
Jens F. Poschet’s present address is: Sandia National Laboratories, Albuquerque, New Mexico, USA.
Elizabeth Perkett’s present address is: Vanderbilt University Medical Center, Nashville, Tennessee, USA.
References
- 1.Rowe S.M., Miller S., Sorscher E.J. Cystic fibrosis. N. Engl. J. Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Mall M., Grubb B.R., Harkema J.R., O’Neal W.K., Boucher R.C. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 3.Stutts M.J., et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 4.Knowles M.R., et al. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J. Clin. Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poschet J., Perkett E., Deretic V. Hyperacidification in cystic fibrosis: links with lung disease and new prospects for treatment. Trends Mol. Med. 2002;8:512–519. doi: 10.1016/s1471-4914(02)02414-0. [DOI] [PubMed] [Google Scholar]
- 6.Poschet J.F., et al. Molecular basis for defective glycosylation and Pseudomonas pathogenesis in cystic fibrosis lung. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13972–13977. doi: 10.1073/pnas.241182598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado M.A., Poschet J.F., Deretic V. Nonclassical pathway of Pseudomonas aeruginosa DNA-induced interleukin-8 secretion in cystic fibrosis airway epithelial cells. Infect. Immun. 2006;74:2975–2984. doi: 10.1128/IAI.74.5.2975-2984.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poschet J.F., et al. Endosomal hyperacidification in cystic fibrosis is due to defective nitric oxide-cylic GMP signalling cascade. EMBO Rep. 2006;7:553–559. doi: 10.1038/sj.embor.7400674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poschet J.F., et al. Hyperacidification of cellubrevin endocytic compartments and defective endosomal recycling in cystic fibrosis respiratory epithelial cells. J. Biol. Chem. 2002;277:13959–13965. doi: 10.1074/jbc.M105441200. [DOI] [PubMed] [Google Scholar]
- 10.Poschet J.F., et al. Pharmacological modulation of cGMP levels by phosphodiesterase 5 inhibitors as a therapeutic strategy for treatment of respiratory pathology in cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L712–L719. doi: 10.1152/ajplung.00314.2006. [DOI] [PubMed] [Google Scholar]
- 11.Di A., et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 12.Pier G.B., et al. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imundo L., Barasch J., Prince A., Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3019–3023. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamo R., Sokol S., Soong G., Gomez M.I., Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am. J. Respir. Cell Mol. Biol. 2004;30:627–634. doi: 10.1165/rcmb.2003-0260OC. [DOI] [PubMed] [Google Scholar]
- 15.Kelley T.J., Drumm M.L. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J. Clin. Invest. 1998;102:1200–1207. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White N.M., et al. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L476–L486. doi: 10.1152/ajplung.00262.2006. [DOI] [PubMed] [Google Scholar]
- 17.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkett E.A., Ornatowski W., Poschet J.F., Deretic V. Chloroquine normalizes aberrant transforming growth factor beta activity in cystic fibrosis bronchial epithelial cells. Pediatr. Pulmonol. 2006;41:771–778. doi: 10.1002/ppul.20452. [DOI] [PubMed] [Google Scholar]
- 19.Vasil M.L., Iglewski B.H. Comparative toxicities of diphtherial toxin and Pseudomonas aeruginosa exotoxin A: evidence for different cell receptors. J. Gen. Microbiol. 1978;108:333–337. doi: 10.1099/00221287-108-2-333. [DOI] [PubMed] [Google Scholar]
- 20.Egan M., et al. Defective regulation of outwardly rectifying Cl- channels by protein kinase a corrected by insertion of CFTR. Nature. 1992;358:581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- 21.Blanchette F., Day R., Dong W., Laprise M.H., Dubois C.M. TGFβ1 regulates gene expression of its own converting enzyme furin. . J. Clin. Invest. 1997;99:1974–1983. doi: 10.1172/JCI119365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broekelmann T.J., Limper A.H., Colby T.V., McDonald J.A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc. Natl. Acad. Sci. U. S. A. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coker R.K., et al. Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am. J. Pathol. 1997;150:981–991. [PMC free article] [PubMed] [Google Scholar]
- 24.Barral A., et al. Transforming growth factor beta as a virulence mechanism for Leishmania braziliensis. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch C.S., Yoneda T., Averill L., Ellner J.J., Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-beta 1. J. Infect. Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 26.Silva J.S., Twardzik D.R., Reed S.G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J. Exp. Med. 1991;174:539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirakata Y., Furuya N., Tateda K., Kaku M., Yamaguchi K. In vivo production of exotoxin A and its role in endogenous Pseudomonas aeruginosa septicemia in mice. Infect. Immun. 1993;61:2468–2473. doi: 10.1128/iai.61.6.2468-2473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaffar-Bandjee M.C., et al. Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa. J. Clin. Microbiol. 1995;33:924–929. doi: 10.1128/jcm.33.4.924-929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inocencio N.M., Moehring J.M., Moehring T.J. Furin activates Pseudomonas exotoxin A by specific cleavage in vivo and in vitro. J. Biol. Chem. 1994;269:31831–31835. [PubMed] [Google Scholar]
- 30.Ogata M., Chaudhary V.K., Pastan I., FitzGerald D.J. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J. Biol. Chem. 1990;265:20678–20685. [PubMed] [Google Scholar]
- 31.Gu M., Gordon V.M., Fitzgerald D.J., Leppla S.H. Furin regulates both the activation of Pseudomonas exotoxin A and the Quantity of the toxin receptor expressed on target cells. Infect. Immun. 1996;64:524–527. doi: 10.1128/iai.64.2.524-527.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H., Iglewski W.J. Cellular ADP-ribosyltransferase with the same mechanism of action as diphtheria toxin and Pseudomonas toxin A. Proc. Natl. Acad. Sci. U. S. A. 1984;81:2703–2707. doi: 10.1073/pnas.81.9.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkmann U., Brinkmann E., Gallo M., Scherf U., Pastan I. Role of CAS, a human homologue to the yeast chromosome segregation gene CSE1, in toxin and tumor necrosis factor mediated apoptosis. Biochemistry. 1996;35:6891–6899. doi: 10.1021/bi952829+. [DOI] [PubMed] [Google Scholar]
- 34.Pastan I., FitzGerald D. Pseudomonas exotoxin: chimeric toxins. J. Biol. Chem. 1989;264:15157–15160. [PubMed] [Google Scholar]
- 35.Koopmann J.O., et al. Export of antigenic peptides from the endoplasmic reticulum intersects with retrograde protein translocation through the Sec61p channel. Immunity. 2000;13:117–127. doi: 10.1016/s1074-7613(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 36.Gordon V.M., Klimpel K.R., Arora N., Henderson M.A., Leppla S.H. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect. Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leitlein J., et al. Processing of immunosuppressive pro-TGF-beta 1,2 by human glioblastoma cells involves cytoplasmic and secreted furin-like proteases. J. Immunol. 2001;166:7238–7243. doi: 10.4049/jimmunol.166.12.7238. [DOI] [PubMed] [Google Scholar]
- 38.Koli K., Saharinen J., Hyytiainen M., Penttinen C., Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc. Res. Tech. 2001;52:354–362. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Perkett E.A., Lyons R.M., Moses H.L., Brigham K.L., Meyrick B. Transforming growth factor-β activity in sheep lung lymph during the development of pulmonary hypertension. J. Clin. Invest. 1990;86:1459–1464. doi: 10.1172/JCI114862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sime P.J., et al. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am. J. Pathol. 1998;153:825–832. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drumm M.L., et al. Genetic modifiers of lung disease in cystic fibrosis. N. Engl. J. Med. 2005;353:1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 42.Wojnarowski C., et al. Cytokine expression in bronchial biopsies of cystic fibrosis patients with and without acute exacerbation. Eur. Respir. J. 1999;14:1136–1144. doi: 10.1183/09031936.99.14511369. [DOI] [PubMed] [Google Scholar]
- 43.Hilliard T.N., et al. Airway remodelling in children with cystic fibrosis. Thorax. 2007 doi: 10.1136/thx.2006.074641. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arkwright P.D., et al. TGF-beta(1) genotype and accelerated decline in lung function of patients with cystic fibrosis. Thorax. 2000;55:459–462. doi: 10.1136/thorax.55.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arkwright P.D., et al. End-organ dysfunction in cystic fibrosis: association with angiotensin I converting enzyme and cytokine gene polymorphisms. Am. J. Respir. Crit. Care Med. 2003;167:384–389. doi: 10.1164/rccm.200204-364OC. [DOI] [PubMed] [Google Scholar]
- 46.Kreiselmeier N.E., Kraynack N.C., Corey D.A., Kelley T.J. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L1286–L1295. doi: 10.1152/ajplung.00127.2003. [DOI] [PubMed] [Google Scholar]
- 47.Kelley T.J., Elmer H.L., Corey D.A. Reduced Smad3 protein expression and altered transforming growth factor-beta1-mediated signaling in cystic fibrosis epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001;25:732–738. doi: 10.1165/ajrcmb.25.6.4574. [DOI] [PubMed] [Google Scholar]
- 48.Vodovotz Y. Control of nitric oxide production by transforming growth factor-beta1: mechanistic insights and potential relevance to human disease. Nitric Oxide. 1997;1:3–17. doi: 10.1006/niox.1996.0105. [DOI] [PubMed] [Google Scholar]
- 49.Corboy M.J., Draper R.K. Elevation of vacuolar pH inhibits the cytotoxic activity of furin-cleaved exotoxin A. Infect. Immun. 1997;65:2240–2242. doi: 10.1128/iai.65.6.2240-2242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratjen F., et al. Diagnostic value of serum antibodies in early Pseudomonas aeruginosa infection in cystic fibrosis patients. Pediatr. Pulmonol. 2007;42:249–255. doi: 10.1002/ppul.20562. [DOI] [PubMed] [Google Scholar]
- 51.Zeitlin P.L., et al. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am. J. Respir. Cell Mol. Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 52.Perez A., Risma K.A., Eckman E.A., Davis P.B. Overexpression of R domain eliminates cAMP-stimulated Cl- secretion in 9/HTEo- cells in culture. Am. J. Physiol. 1996;271:L85–L92. doi: 10.1152/ajplung.1996.271.1.L85. [DOI] [PubMed] [Google Scholar]
- 53.Zabner J., et al. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L844–L854. doi: 10.1152/ajplung.00355.2002. [DOI] [PubMed] [Google Scholar]
- 54.Cozens A.L., et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 55.Gruenert D.C., et al. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajan S., et al. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am. J. Respir. Cell Mol. Biol. 2000;23:304–312. doi: 10.1165/ajrcmb.23.3.4098. [DOI] [PubMed] [Google Scholar]
- 57.Molloy S.S., Bresnahan P.A., Leppla S.H., Klimpel K.R., Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- 58.Abe M., et al. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 59.Sarac M.S., Cameron A., Lindberg I. The furin inhibitor hexa-D-arginine blocks the activation of Pseudomonas aeruginosa exotoxin A in vivo. Infect. Immun. 2002;70:7136–7139. doi: 10.1128/IAI.70.12.7136-7139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laithwaite J.E., Benn S.J., Yamate J., FitzGerald D.J., LaMarre J. Enhanced macrophage resistance to Pseudomonas exotoxin A is correlated with decreased expression of the low-density lipoprotein receptor-related protein. Infect. Immun. 1999;67:5827–5833.. doi: 10.1128/iai.67.11.5827-5833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.