Abstract
COL27A1 is a member of the collagen fibrillar gene family and is expressed in cartilaginous tissues including the anlage of endochondral bone. To begin to understand its role in skeletogenesis, the temporospatial distributions of its RNA message and protein product, type XXVII collagen, were determined in developing human skeletal tissues. Laser capture microdissection and quantitative reverse-transcription polymerase chain reaction demonstrated that gene expression occurred throughout the growth plate, and that it was higher in the resting and proliferative zones than in hypertrophic cartilage. Immunohistochemical analyses showed that type XXVII collagen was most evident in hypertrophic cartilage at the primary ossification center and at the growth plate, and that it accumulated in the pericellular matrix. Synthesis of type XXVII collagen overlapped partly with that of type X collagen, a marker of chondrocyte hypertrophy, preceded the transition of cartilage to bone, and was associated with cartilage calcification. Immunogold electron microscopy of extracted ECM components from mouse growth plate showed that type XXVII collagen was a component of long non-banded fibrous structures, filamentous networks, and thin banded fibrils. The timing and location of synthesis suggest that type XXVII collagen plays a role during the calcification of cartilage and the transition of cartilage to bone.
Keywords: COL27A1, type XXVII collagen, cartilage, chondrocyte, growth plate, bone, endochondral, skeletogenesis
INTRODUCTION
COL27A1 encodes a proalpha chain of type XXVII collagen and joins a family of 42 other genes whose protein products combine to form at least 28 distinct trimeric collagen molecules [1, 2]. COL27A1 was identified by an interrogation of databases for collagen-like motifs, and its complete sequence and structure were determined by a combination of bioinformatic and molecular approaches [3]. The sequence and arrangement of motifs are similar to those seen in COL24A1 [4], another fibrillar collagen gene, and together, they are most similar to COL5A1, COL5A3, COL11A1, and COL11A2 among the fibrillar collagen genes. Like these genes, COL27A1 encodes a long triple helical domain, a carboxyl-terminal propeptide (C-propeptide), and a large globular amino-terminal propeptide (N-propeptide). However, the predicted proα1(XXVII) chain differs from these closely related fibrillar collagen chains in that there is no minor triple helix, the major triple helical domain is shorter, and there are two short interruptions in the characteristic collagen Gly-Xaa-Yaa repeating triplet motif of the triple helical domain [3].
Expression of mouse col27a1 in 14.5-day embryos was most abundant in cartilaginous tissues, including the anlage of long bones, ribs, and spine, as well as in the eye and otic capsule, in a pattern similar to that of the type II and XI collagen genes [3]. At 19 days gestation, expression was prominent in the nasal cartilages and at low levels in elements of the gastrointestinal tract and tooth-forming cells [5]. In human tissues, expression was identified in long bone anlage of the hand derived from 10.8-week embryos, in trachea, lung, and skin at 12.4 weeks gestation. Expression was also detected in the mucosal layer of the stomach in fetal human tissues at 15.3 weeks gestation [5].
COL27A1 is expressed at 18 to 20-weeks gestation in human fetal epiphyseal cartilage where message represents approximately 0.14% of total transcripts and 1.15% of all collagen transcripts [6]. Levels were lower than those of cartilage collagen genes COL2A1, COL9A1, COL11A1, COL9A3, COL9A2, and COL11A2, but higher than that of COL10A1, whose expression is restricted to hypertrophic zones. A similar study using human growth plate cartilage from 20 week prenatal to 2 year postnatal samples determined that COL27A1 transcripts represented 0.05% of total and 1.1% of collagen transcripts, and again more than that of COL10A1 [7]. Because of its expression in cartilage, COL27A1 was screened for enhancer elements that regulate transcription of other collagen genes in cartilage, and two paired SOX9-responsive elements, typical of those found in other cartilage collagen genes, were identified in intron [8].
Most of the mammalian skeleton is formed by means of a cartilage intermediate, in which chondrocytes become hypertrophic, manufacture a specialized extracellular matrix (ECM), mediate calcification of that matrix, and undergo apoptosis. Blood vessels invade from the perichondrium and with them come bone cells [9]. These cells – osteoblasts and osteoclasts –initiate the processes of regular bone formation and remodeling. This series of events begins at the primary ossification center, proceeds toward the ends of the presumptive bone and continues in the growth plate. Based on our previous studies [3, 8], we expected type XXVII collagen to be found during the cartilage stages of bone development. To test this hypothesis, we examined the distribution of type XXVII collagen and COL27A1 mRNA in developing endochondral bone. These observations define the pattern of expression and suggest that type XXVII collagen plays a role in the transition of cartilage to bone during skeletogenesis.
MATERIALS AND METHODS
Antibodies
Proteintech (Chicago, IL) synthesized an antigenic peptide [CSQTPLVPAKQSARKTP, residues 324–339 (counting from the initiator methionine)] from the N-propeptide domain of proα1(XXVII) and made a rabbit antibody against it. The peptide was selected from rat sequence that was similar to those of mouse and human proα1(XXVII) chains, but distinct from sequences in other fibrillar procollagens. Antibodies were affinity purified from serum and cross-absorbed with the antigenic peptides to remove unwanted cross-reactivities. Staining of developing skeletal tissues was not observed after incubation with pre-immune serum (data not shown).
Two antibodies were obtained from the Developmental Studies Hybridoma Bank at the University of Iowa: anti-collagen type I (M-38) and anti-collagen type II (6B3). A polyclonal antibody against type X collagen was a gift from Dr. David Eyre, Department of Orthopedics, University of Washington.
Immunohistochemistry
Some human fetal tissue used in this study was acquired from the Birth Defects Research Laboratory at the University of Washington with the approval of the Institutional Review Board for Human Subjects (IRB # 961826-A12). Tissues were obtained from 57-day and 67-day fetuses and middle phalanges of the hand were used in the study. Estimated gestational age was determined from maternal histories and fetal crown-rump measurements.
Tissues were immediately placed in O.C.T compound and snap frozen in an ethanol/dry ice slush. Six μm cryo-sections were cut using a cryostat, dried for 5 minutes on a 37ºC hotplate and then placed in a vacuum with desiccant overnight. The sections were fixed in acetone at −20ºC, allowed to dry for 5 minutes and then rinsed in TBS. The sections were then incubated in serum (1.3% goat serum, 0.1% bovine serum albumin in TBS) for 30 minutes. Sections were next incubated with primary antibody [either monoclonal mouse anti-collagen I (1:175), monoclonal mouse anti-collagen II (1:100), rabbit anti-collagen X (1:1000), or rabbit anti-type XXVII collagen (1:40)], diluted with TBS containing 1.5% normal goat serum and 1mg/ml BSA for 1 hour at room temperature in a humid chamber. The sections were rinsed in TBS and then incubated with serum for five minutes. Sections were incubated with either donkey anti-mouse TRED (1:300) or donkey anti-rabbit FITC (1:200) (Jackson ImmunoResearch Laboratories, West Grove, PA) in diluent for 30 minutes at room temperature in a humid chamber. Sections were rinsed in TBS and incubated with nuclear marker 4,6 diamidino-2-phenylindole (DAPI, Sigma, St. Louis, MO) for 5 minutes at room temperature, rinsed in distilled water and cover-slipped with Prolong anti-fade mounting media (Molecular Probes, Carlsbad, CA).
To mark calcium deposition, sections were treated for one minute with 1% Alizarin Red dye, counterstained for 30 seconds with hematoxylin, rinsed and cover-slipped with glycergel (DAKO, Glostrup, Denmark) at 55ºC.
To unmask potentially hidden type XXVII collagen epitopes, sections were treated with 1) 0.1% hyaluronidase (Sigma, St. Louis, MO) in PBS at 37°C for 30 minutes; 2) MMP-13 (Biomol, Plymouth Meeting, PA; 0.5, 0.05 or 0.005 μg/section) at 37°C for up to 18 hours; 3) 6 M urea (pH 4.8) at 50°C for 25 minutes; 4) 0.01% trypsin in TBS for five minutes; 5) 0.3% SDS in 0.05M tris buffer for 10 minutes; 6) heated 10mM citrate buffer for 2 minutes; or 7) 0.1% Tween 20 (Bio-Rad Laboratories, Hercules, CA) in 1.3% goat serum and 0.1% BSA. These tissues were examined with a fluorescence Nikon Microphot microscope.
Distal femur growth plate from a normal 20-week fetus was formalin fixed and then decalcified in 3% EDTA diluted in 0.1M PBS. Sections were incubated for one hour in 5% goat serum and 0.1% triton in PBS and then incubated with anti-collagen XXVII at a 1:40 dilution in 5% serum in PBS at 4°C for 3 hours. Sections were washed and treated with a secondary antibody labeled with Alexa Fluor 568 goat anti-rabbit IgG (Molecular Probes, Eugene, OR) at a 1:200 dilution in 5% serum in PBS at room temperature for 1 hour. The sections were washed and mounted on slides using VECTASHIELD (Vector Laboratories, Burlingame, CA) with DAPI. Duplicates of each section were incubated with phosphate-buffered saline instead of the primary antibody as a negative control. These tissues were examined with a Leica TCS SP confocal microscope.
Immunogold electron microscopy
Three-day-old mice were euthanized and cartilaginous growth plates were dissected from true ribs and immediately frozen on dry ice. Slices of tissue were homogenized in small volumes of phosphate buffered saline (PBS), pH 7.4, containing a mixture of protease inhibitors, and homogenates were cleared by low speed centrifugation.
Immunogold electron microscopy was performed as described [10, 11] with some modification. Aliquots of extracts from mouse rib cartilage were adsorbed to nickel grids coated with Formvar/carbon. Extracts were incubated for 2 hours with the anti-type XXVII collagen antibody diluted 1:100 in 0.25% (w/v) skim milk in PBS or with a mouse monoclonal antibody against collagen II (MAB 8887; Chemicon, Temecula, CA) diluted 1:100 in the same buffer. After washing with PBS, the grids were incubated with goat antibodies to rabbit and mouse immunoglobulins conjugated to 12 nm and 18nm colloidal gold particles diluted 1:30 (Jackson ImmunoResearch Laboratories, West Grove, PA). Grids were washed and stained with 2 % uranyl acetate. Control experiments were done with the primary antibodies omitted. In some experiments, to unmask potentially hidden type XXVII collagen epitopes, the fibrillar extracts were treated with 1) hyaluronidase from bovine testes (Sigma, St. Louis, MO) for 1 hour at 37°C; 2) 4 M GuHCl for 2 hours at room temperature; 2) 6 M urea at 50°C for 10 minutes, 1, 2 or 4 hours; 3) MMP-13 (Invitec, Germany) according to the manufacture’s instructions at 37°C for 1, 2, 4 or 24 hours; and 4) 0.01 % trypsin in TBS for 1 or 2 hours before the immunogold labelling. Electron micrographs were taken at 60 kV with a Philips EM-410 electron microscope.
Laser capture microdissection and quantitative reverse transcription-polymerase chain reaction
A normal middle phalanx from the toe of a 7-month old child with hemimelia that had been surgically removed, was placed in RNAlater™ (Ambion, Austin, TX), and stored at −80ºC. The sample was processed for laser capture microdissection and quantitative reverse transcription polymerase chain reaction (QRT-PCR) as described [12]. Twenty-five sections (5 μm) were microdissected using a Pixcell II laser capture microscope (Arcturus Engineering, Mountain View, CA). Groups of chondrocytes were captured from the resting/proliferative zone and the hypertrophic zone of the growth plate. Cells captured on PixCell cap surfaces were lysed with a guanidine thiocyanate buffer, and RNA was isolated, DNase-treated and reverse-transcribed.
Human-specific primers for COL27A1 were designed to span an intron using Primer Express 1.0 software (Applied Biosystems, Foster City, CA). The primer sequences were 5’GGTCTCCTGCAACTTCACTCAT3’ (nucleotides 5157–5178, from the A of the initiator methionine codon) and 5’GCTTAGCAGGTGCAGGAAATTC3’ (nucleotides 5247–5268). Gene expression was analyzed with an ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA). For QPCR analysis, each sample contained Sybr Green Master Mix (Applied Biosystems, Foster City, CA), 0.3 μM of each primer and cDNA. Appropriate negative controls of buffer blanks and no-templates were added to confirm the absence of DNA contamination and possible primer:dimer formation. Dissociation curves were generated with the software program v1.Ob.1 (Applied Biosystems, Foster City, CA) to verify the correct melting temperatures for the amplicon products.
Standard curves were constructed in this study from stock RNA isolated from human articular chondrocytes grown to confluence in vitro. Serial dilutions were prepared of the cDNA generated from the stock RNA. Triplicate threshold cycle (Ct) values were produced for each serial dilution. QPCR conditions were (Stage 1) 95ºC for 10 min and (Stage 2) 40 cycles at 95ºC for 15 sec, 60ºC for 30 sec, and 72ºC for 2 min. Following the methodology for relative standard curves (User Bulletin #2, Applied Biosystems), plots of log input concentration (ng) vs Ct for each gene were obtained. Expression levels of COL27A1 were normalized to the reference gene, 18S rRNA. Amplicon products were resolved on a 3% Sybr Gold-stained agarose gel. Images were generated and stored utilizing a Typhoon model 8610 Scanner (GE Healthcare, Piscataway, NJ).
RESULTS
Type XXVII collagen was detected at the bone primary ossification center
Type XXVII collagen was identified in developing human phalanges by immunohistochemistry (Fig. 1). To provide a developmental context, neighboring serial sections were stained with antibodies to other collagens, types I, II, and X, or with alizarin red, a dye that binds calcium as an indicator of cartilage calcification.
Figure 1. Temporospatial distribution of type XXVII collagen in the primary ossification center.
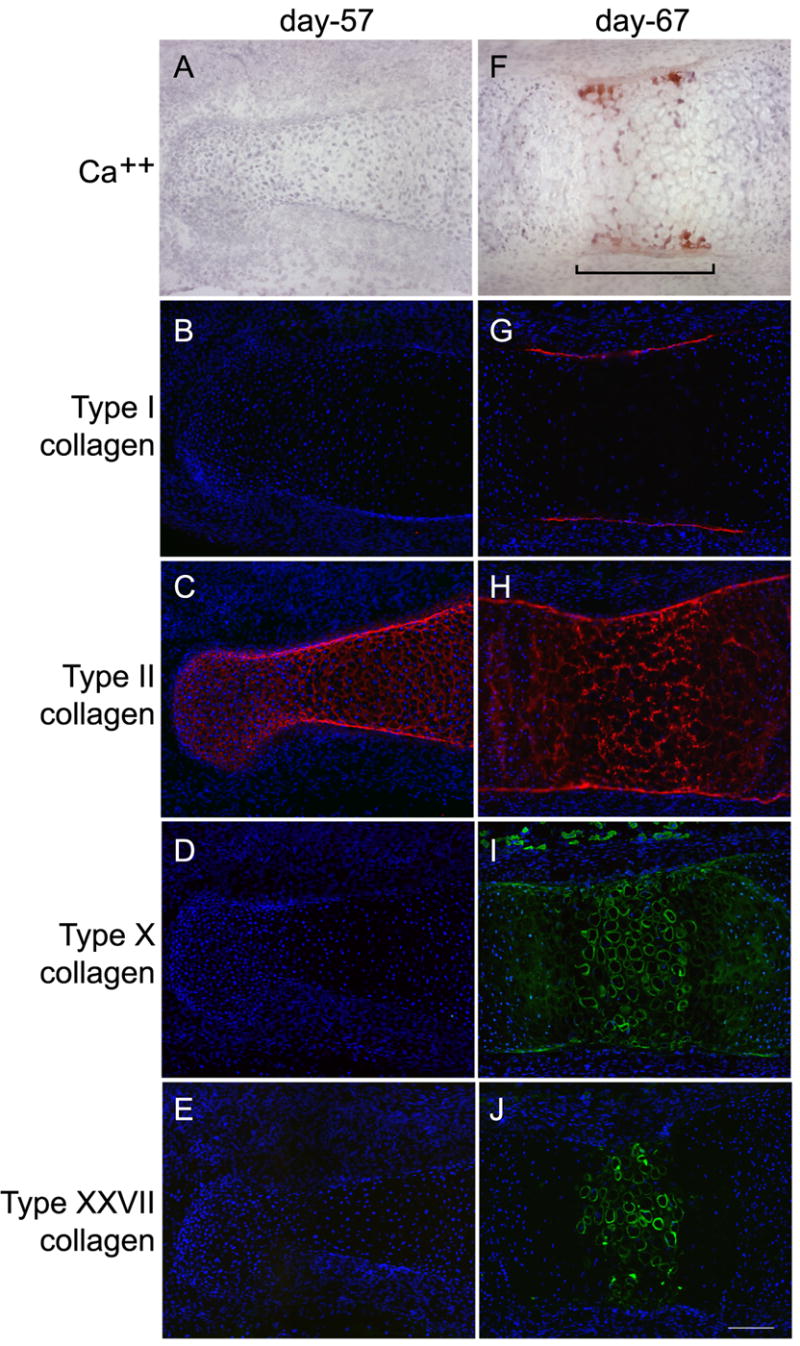
Cartilaginous primordial middle phalanges from 57- and 67-day human fetal fingers were sectioned and stained with alizarin red, which binds calcium (A, F), and with antibodies directed against collagen types I (B, G), II (C, H), X (D, I), and XXVII (E, J). At day 57, there was no cartilage calcification and, of the four collagens, only type II (the predominant cartilage collagen) was present. At day 67, cells at the primary ossification center (bracket) were hypertrophic and the matrix surrounding these cells was calcified. Type I collagen (the main protein of bone) was detected only in the perichondrium. Type II collagen remained throughout the structure. Type X collagen was prominent in the ECM surrounding hypertrophic cells. Type XXVII collagen was detected in the ECM around hypertrophic cells and its pattern of distribution overlapped, in part, with that of calcification and type X collagen. Magnification bar is 125μM.
At embryonic day 57 (Fig. 1A–E), the cartilage model of bone was fully formed and the chondrocytes in the center of the future diaphysis were increased in size (relative to cells in adjacent zones), evidence of progression towards hypertrophy. No calcium deposition was detected at this stage of development (Fig 1E). The major cartilage collagen, type II, was observed throughout the structure (Fig 1C). Type I collagen, the principal protein component of bone (Fig. 1B); type X collagen, found in hypertrophic cartilage (Fig. 1D); and type XXVII (Fig 1E) collagen were not detected.
By embryonic day 67 (Fig. 1F–J), the cartilage in the center of the future diaphysis was calcified (Fig. 1F). Chondrocytes were hypertrophic and the extracellular space around them was reduced. Type II collagen remained ubiquitously deposited throughout the structure, though its distribution at the primary ossification center was altered, reflecting the changes in cell size and ECM breadth (Fig. 1H). Type I collagen was restricted to the perichondrium and future bone collar at the primary ossification center (Fig. 1G). Type X collagen was localized immediately around cells, around lacunae from which cells were missing (indicated by lack of nuclear DAPI stain), and diffusely in regions flanking the newly calcified cartilage (Fig. 1I). Type XXVII collagen was detected in the primary ossification center and was tightly restricted to the pericellular region of the hypertrophic chondrocytes and lacunae at the very center of the future diaphysis (Fig 1J).
Treatment with testicular hyaluronidase (degrades hyaluronic acid), MMP-13 (cleaves type II collagen), urea (denatures collagens), reduced background noise and increased the signal at the primary ossification center, but did not increase the range or distribution of type XXVII collagen staining. Incubation with other potential unmasking agents – trypsin, SDS, citrate buffer, or Tween 20 – had no effect (data not shown).
Type XXVII collagen was detected at the growth plate
Immunohistochemical analyses of fetal 20-week human femoral growth plates showed that type XXVII collagen was in greatest abundance in the hypertrophic zone at the chondro-osseous junction (Fig. 2A). There was some staining detected around cells in the resting (Fig. 2B) and proliferative (Fig. 2C) zones of the cartilaginous plate, but the most intense staining occurred deep in the hypertrophic zone near the newly formed bone. Type XXVII collagen was seen throughout the ECM in this zone, and as observed in the primary ossification center, it was also closely situated around hypertrophic chondrocytes (Fig. 2D).
Figure 2. Localization of type XXVII collagen in the growth plate.
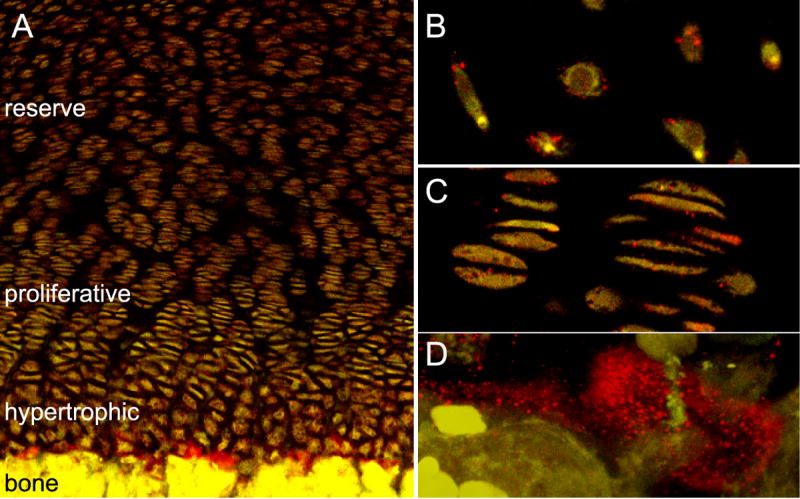
Tissue from a 20-week-old human femoral growth plate was sectioned and interrogated with an antibody directed against type XXVII collagen (red). Type XXVII collagen was detected in the three cartilaginous zones: hypertrophic, proliferative, and reserve. However, staining was most intense in the hypertrophic region, at the interface of newly deposited bone and cartilage (A). Higher magnification of reserve (B) and proliferative (C) zones showed type XXVII collagen on the cell surfaces. Increased magnification of the hypertrophic zone at the cartilage-bone boundary showed type XXVII collagen surrounding chondrocytes (D).
Type XXVII collagen was detected in several distinct structures of the ECM
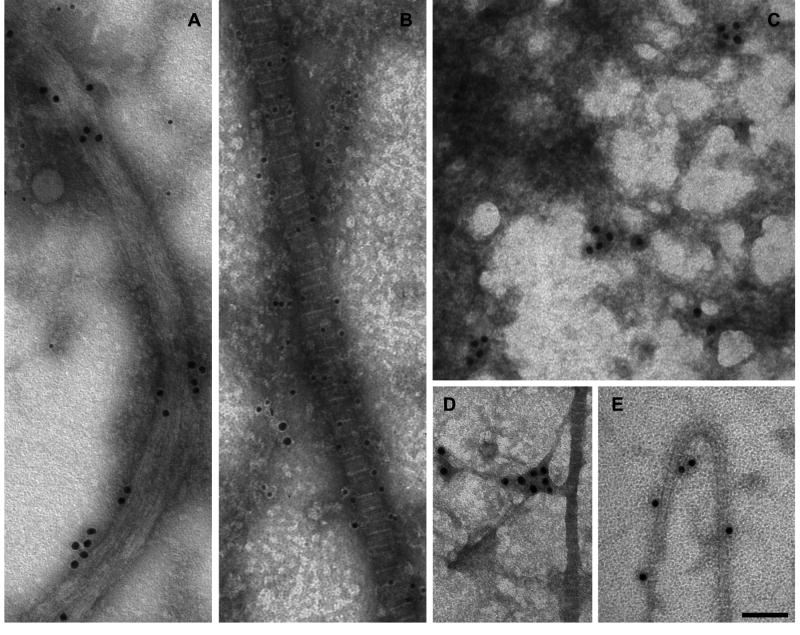
The physical relationship of type XXVII collagen with supramolecular structures of the ECM was examined by immunogold transmission electron microscopy. We did not see staining in the human tissue for unknown reasons, but type XXVII collagen was detected in extracted extracellular components of growth plate from mouse neonates (Fig. 3). Collagen XXVII antibodies reacted with three distinct structures: 1) non-banded, fibrous structures about the same size and shape as type II collagen-containing fibrils; 2) amorphous filamentous mat-like formations; and 3) thin, banded collagen fibrils.
Figure 3. Ultrastructural localization of type XXVII collagen in the extracellular matrix.
Components of ECM were extracted from mouse growth plate cartilage and analyzed by immunogold transmission electron microscopy. Figures A and B show extracts that were dual-labeled with antibodies against type II collagen (12 nm particles) and type XXVII collagen (18 nm particles). Figures C–E show single label-staining with an antibody against type XXVII collagen (18 nm particles). (A) Type XXVII collagen was detected in fibrous, non-banded structures of width and length similar to that of banded collagen fibrils. These structures were devoid of type II collagen. (B) Large banded collagen fibrils showed heavy type II collagen staining and no type XXVII collagen labeling. (E) Type XXVII collagen was detected on some smaller banded fibrils. (C) Type XXVII collagen was detected in filamentous, amorphous mesh-works that were sometimes associated with the extracted collagen fibrils (D). Bar = 100 nm.
Antibodies to type XXVII collagen labeled fibrous structures, about 80 nm in width, as clusters of three to five gold particles and was accentuated by urea and GuHCl treatment (Fig. 3A). Type II collagen was not a part of this structure. Type XXVII collagen labeling was detected on some small banded collagen fibrils (Fig. 3E), but it was scattered along the fibrils, often with long unlabeled intervals, and a clear relationship between the gold particles and the periodic banding pattern of fibrils was not observed. Some type II collagen-containing fibrils had no detectable type XXVII collagen labeling at all (Fig. 3B). The electron-dense filamentous mats were labeled by type XXVII collagen antibodies, but no clear pattern of antibody binding was evident (Fig. 3C), and it was sometimes associated with fibrils (Fig. 3D). Dual-labeling (Figs. 3A and 3B) indicated that collagens XXVII and II were part of different structures.
COL27A1 mRNA was differentially distributed in the growth plate
To determine the precise distribution of COL27A1 mRNA in cartilage elements of skeletal tissues, the growth plate of a human supernumerary toe was examined by laser capture microdissection and QRT-PCR. The characteristic tissue morphology and laser capture microdissection of the phalangeal growth plate are evident in a series of eosin-stained sections (Fig. 4). The images illustrate an area of the growth plate containing resting, proliferating, and hypertrophic chondrocytes (Fig. 4A), some of which were identified by laser capture (Fig. 4B) and then removed from the section and isolated on a Capsure transfer film (Fig. 4C) for subsequent molecular analysis. In a similar manner, other hypertrophic and resting/proliferative cells were captured and analyzed. 18S rRNA was used to assure the quality of RNA isolated from the various chondrocytes of the growth plate, and utilized as the normalizing gene for QRT-PCR. The Sybr Gold-stained gel (Fig. 4D) verified the presence of 18S rRNA and COL27A1 message in both resting/proliferative chondrocytes and in hypertrophic cells, and showed the relative abundance of COL27A1 mRNA in each zone. The normalized threshold cycle (Ct) values indicated that COL27A1 message was 16 times more abundant in resting/proliferative cells than in hypertrophic cells.
Figure 4. Laser capture microdissection of a human growth plate and QRT-PCR of COL27A1.
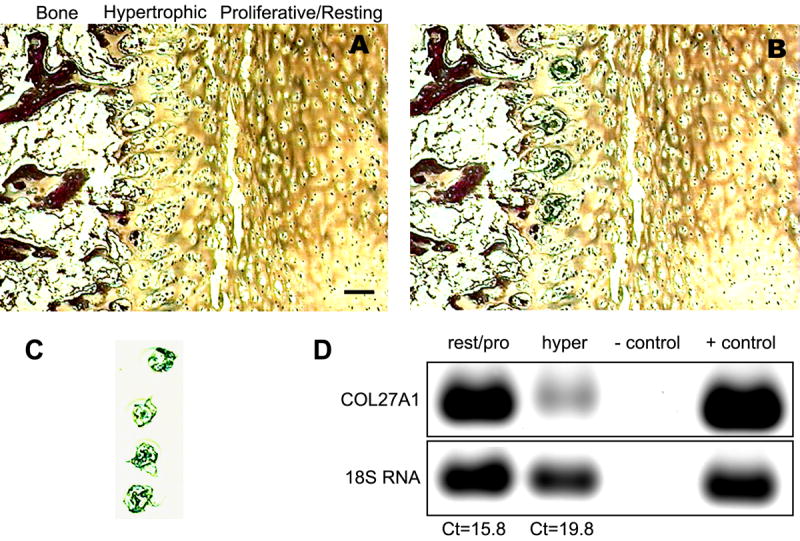
An eosin-stained frozen section of growth plate cartilage from a 7-month-old human phalanx demonstrated laser capture of hypertrophic zone chondrocytes. The tissue is shown before (A) and after (B) laser activation melted polymer around the cells of interest. The microdissected hypertrophic cells attached to the polymer film substrate are shown in panel C. Bar = 100 μm for panels A–C. Captured cells were used to obtain Sybr Gold-stained gels and QRT-PCR of COL27A1 and 18S rRNA (D). Reaction buffers without cDNA served as a negative control. RNA isolated from human articular chondrocytes grown in vitro was used as a positive control. Normalized to 18S rRNA, levels of COL27A1 were 16-fold greater [difference of 4 threshold cycles (Cts)] in resting/proliferative cells than in hypertrophic chondrocytes.
DISCUSSION
Both we and a second group had noted that the principal and apparently robust expression of the mouse col27a1 gene was in the cartilaginous tissues throughout the body at 14.5 days gestation [3, 5]. On the basis of those findings, we expected that type XXVII collagen would be ubiquitously distributed throughout cartilage and that it may play a role in formation of the type II collagen-containing fibrils or in other functions of the matrix. Instead, the major site of type XXVII collagen deposition is in skeletal tissues at sites of transition from cartilage to bone. In developing long bone at the primary ossification center and at the epiphysis at 20-weeks gestation in the human, type XXVII collagen appears as a recognizable antigen concurrent with mineralization of the cartilage and just prior to invasion of bone cells.
The protein often surrounds, what appears to be, lacunae that may have held differentiated hypertrophic chondrocytes that underwent apoptosis. At the primary ossification center, the cartilage was partially calcified and suspected lacunae were demarcated by type XXVII collagen. At those sites, type XXVII collagen was restricted to the center of the mineralizing zone and to the region in which chondrocytes were apoptotic. In the growth plate, type XXVII collagen was present at cell surfaces in the resting zone, proliferative zone, and the hypertrophic zone where it was most abundant. High levels of protein at the deepest levels of the hypertrophic zone may therefore be explained by a continual accumulation of collagen in the pericellular region over the life of the chondrocyte, from resting through hypertrophic stages.
These findings suggest that type XXVII collagen plays a role in later stages of the cartilage modeling phase of endochondral bone formation. These developmental events include cartilage mineralization, apoptosis of chondrocytes and formation of acellular spaces, invasion of blood vessels, introduction of bone cells, degradation of the cartilage matrix, deposition of a type I collagen-rich ECM, and formation of bone. In bone, type I collagen serves as a template for organized mineral nucleation and growth [13]. Type XXVII collagen is a good candidate for a scaffold of mineralization in cartilage and as the supporting environment for invading blood vessels. Little is known about the formation, stabilization, and function of lacunae left by apoptotic chondrocytes, but it is believed that they provide space for vascularization of the calcified matrix. Type XXVII collagen encapsulates the lacunae, and we think it could provide a calcified framework that supports the cavities into which vessels grow.
Before chondrocytes become hypertrophic they synthesize collagen types II, IX and XI, which assemble into heterotypic fibrils [14]. During hypertrophy, production of these collagens decreases and type X collagen is made, which is thought to assemble into filamentous mat-like structures and onto the surface of collagen fibrils [15]. Our study shows that type XXVII collagen is synthesized and secreted by a similar set of cells that produce type X collagen and suggest that the two molecules could interact in the ECM. The immunogold electron microscopy data show that type XXVII collagen is found in filamentous meshworks that are similar to those described in relation to type X collagen and comprises part of some banded collagen fibrils. However, type XXVII collagen was found most prominently in non-banded fibrous structures that were devoid of type II collagen. Other components and the function of these structures remain unknown.
During the revision of this manuscript, a paper by Plumb and colleagues [16] on type XXVII collagen in developing mouse was published. Observations from that and the present study are consistent: type XXVII collagen is present in both mouse and human skeletal elements at similar developmental times and places. These two studies also showed that type XXVII collagen is part of long, non-banded fibrils from which type II collagen is excluded. Though they appear to be the same structure in each paper, there is disagreement on the width (10 nm in the report by Plumb et al. versus 80 nm in the present study). Size of these fibrils may be naturally variable or the observed variation could be explained by different sample isolation and preparation methods.
Three hundred seventy-two distinct genetic skeletal disorders are currently recognized [17]. Of these, 215 are linked to mutations in one or more genes, leaving 157 diseases for which the genetic bases remain unknown. These figures show two things about skeletogenesis. First, the process entails complex genetic interactions and, second, though considerable progress has been made in recent decades, our understanding of bone development and the molecular pathogenesis of its disorders remains incomplete. Collagens are the most abundant proteins in skeletal tissues and mutations in the encoding genes result in a significant fraction of these diseases. Based on our findings, it is probable that type XXVII collagen, like other members of its family, plays a significant role in bone development. We performed genetic linkage analysis or sequenced COL27A1 from people with several candidate disease phenotypes: Schmid metaphyseal dysplasia (unlinked to COL10A1), metaphyseal anadysplasia, spondyloepiphyseal dysplasia with amelogenesis imperfecta, acrolaryngeal syndrome, Stickler syndrome (unlinked to COL2A1, COL9A1, or COL11A1) and osteogenesis imperfecta with amelogenesis imperfecta (unlinked to COL1A1 or COL1A2). No linkage to or mutations in COL27A1 were found (data not shown). These negative findings and the other observations from this study should narrow the search for a linked human disease and provide clues about the biological function of type XXVII collagen.
Acknowledgments
The Birth Defects Research Laboratory at the University of Washington is support by NIH HD 000836. UH is supported in part by the Sonderforschungsbereich (SFB) 492 project A2 to PHB at the University Hospital Muenster, Germany. JMP is supported in part by NIH AR052476. RJF is supported in part by NIH AR052896. DK is supported by HD22567. HET is supported by a National Science Foundation Graduate Research Fellowship. Molecular analysis by laser capture microdissection and QRT-PCR is supported by NIH AR 41452 to WJL. We thank Laura Bridgewater and Jaime Mayo of Brigham Young University for useful discussion and critique, and Ms. G. Pentrup for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Veit G, Kobbe B, Keene DR, Paulsson M, Koch M, Wagener R. Collagen XXVIII, a novel VWA-domain-containing protein with many imperfections in the collagenous domain. J Biol Chem. 2005 doi: 10.1074/jbc.M509333200. [DOI] [PubMed] [Google Scholar]
- 2.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Pace JM, Corrado M, Missero C, Byers PH. Identification, characterization and expression analysis of a new fibrillar collagen gene, COL27A1. Matrix Biol. 2003;22:3–14. doi: 10.1016/s0945-053x(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 4.Koch M, Laub F, Zhou P, Hahn RA, Tanaka S, Burgeson RE, Gerecke DR, Ramirez F, Gordon MK. Collagen XXIV, a vertebrate fibrillar collagen with structural features of invertebrate collagens: selective expression in developing cornea and bone. J Biol Chem. 2003;278:43236–44. doi: 10.1074/jbc.M302112200. [DOI] [PubMed] [Google Scholar]
- 5.Boot-Handford, R. P., Tuckwell, D. S., Plumb, D. A., Rock, C. F., and Poulsom, R. A novel and highly conserved collagen (pro(alpha)1(XXVII)) with a unique expression pattern and unusual molecular characteristics establishes a new clade within the vertebrate fibrillar collagen family. J Biol Chem. 2003;278:31067–77. doi: 10.1074/jbc.M212889200. [DOI] [PubMed] [Google Scholar]
- 6.Pogue R, Sebald E, King L, Kronstadt E, Krakow D, Cohn DH. A transcriptional profile of human fetal cartilage. Matrix Biol. 2004;23:299–307. doi: 10.1016/j.matbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Tagariello A, Schlaubitz S, Hankeln T, Mohrmann G, Stelzer C, Schweizer A, Hermanns P, Lee B, Schmidt ER, Winterpacht A, Zabel B. Expression profiling of human fetal growth plate cartilage by EST sequencing. Matrix Biol. 2005;24:530–8. doi: 10.1016/j.matbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins E, Moss JB, Pace JM, Bridgewater LC. The new collagen gene COL27A1 contains SOX9-responsive enhancer elements. Matrix Biol. 2005;24:177–84. doi: 10.1016/j.matbio.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 10.Kassner A, Hansen U, Miosge N, Reinhardt DP, Aigner T, Bruckner-Tuderman L, Bruckner P, Grassel S. Discrete integration of collagen XVI into tissue-specific collagen fibrils or beaded microfibrils. Matrix Biol. 2003;22:131–43. doi: 10.1016/s0945-053x(03)00008-8. [DOI] [PubMed] [Google Scholar]
- 11.Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989;108:191–7. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacquet R, Hillyer J, Landis WJ. Analysis of connective tissues by laser capture microdissection and reverse transcriptase-polymerase chain reaction. Anal Biochem. 2005;337:22–34. doi: 10.1016/j.ab.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Su X, Sun K, Cui FZ, Landis WJ. Organization of apatite crystals in human woven bone. Bone. 2003;32:150–62. doi: 10.1016/s8756-3282(02)00945-6. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes RJ, Schmid TM, Eyre DR. Assembly of collagen types II, IX and XI into nascent hetero-fibrils by a rat chondrocyte cell line. Eur J Biochem. 2003;270:3243–50. doi: 10.1046/j.1432-1033.2003.03711.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmid TM, Linsenmayer TF. Immunoelectron microscopy of type X collagen: supramolecular forms within embryonic chick cartilage. Dev Biol. 1990;138:53–62. doi: 10.1016/0012-1606(90)90176-j. [DOI] [PubMed] [Google Scholar]
- 16.Plumb DA, Dhir V, Mironov A, Ferrara L, Poulsom R, Kadler KE, Thornton DJ, Briggs MD, Boot-Handford RP. Collagen XXVII is developmentally regulated and forms thin fibrillar structures distinct from those of classical vertebrate fibrillar collagens. J Biol Chem. 2007;282:12791–5. doi: 10.1074/jbc.C700021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Superti-Furga A, Unger S. Nosology and classification of genetic skeletal disorders: 2006 revision. Am J Med Genet A. 2007;143:1–18. doi: 10.1002/ajmg.a.31483. [DOI] [PubMed] [Google Scholar]