Abstract
Maternal treatment with corticosteroids reduces blood-brain barrier permeability in premature ovine fetuses and the incidence of intraventricular hemorrhage in premature infants. We tested the hypothesis that maternally administered corticosteroids increase the expression of tight junction (TJ) proteins in the cerebral cortex of ovine fetuses with and without exposure to in-utero brain ischemia. Fetuses at 80% of gestation were studied 18 h after the last of four 4−6 mg dexamethasone or placebo injections were given over 48 h to ewes. Groups were placebo/control, dexamethasone/control, placebo/ischemic, and dexamethasone/ischemic. Ischemia consisted of 30 min of fetal carotid artery occlusion and 72 h of reperfusion. Cerebral cortex was snap frozen. Western immunoblot was used to measure the protein expression of occludin, claudin-1, claudin-5, zonula occludens (ZO)-1, and ZO-2, and a TJ accessory protein annexin II. Occludin and annexin II protein expression were 48% and 58% higher (P<0.05) in the dexamethasone/ischemic than placebo/control group, respectively. Claudin-5 protein expression was 69% and 73% higher (P<0.05) in the placebo/ischemic and dexamethasone/ischemic than placebo/control group. Claudin-1 expression did not differ among groups. ZO-1 protein expression was 25%, 40%, and 55% lower in the dexamethasone/control, placebo/ischemic and dexamethasone/ischemic than placebo/control group, respectively. ZO-2 expression was 45% and 70% lower (P<0.01) in the placebo/ischemic and dexamethasone/ischemic than placebo/control group. We conclude that maternal corticosteroid treatment differentially regulates the expression of component proteins of TJs in the cerebral cortex of fetuses exposed to brain ischemia. The functional significance of this differential regulation warrants further investigation.
1. Introduction
The blood-brain barrier (BBB) is composed of a continuous layer of cerebrovascular endothelial cells connected by tight intercellular junctions [4]. TJs are the main structures responsible for the properties of the BBB [21]. They are composed of transmembrane proteins such as occludin, claudins, and junctional associated proteins that seal the inter-endothelial space between adjacent endothelial cells [21]. Cytoplasmic proteins such as ZO-1 and ZO-2 are associated with TJs, located at the cytoplasmic surface of endothelial cells and connect TJs to actin and other cytoskeletal proteins [21].
Transmembrane proteins are synthesized in the cytoplasm, incorporated into the lipid bilayer of vesicles that then fuse with cell membranes, and traffic these proteins into the membranes [21 5]. Annexins and other accessory proteins enhance the fusion of the lipid bilayer in the cell [27].
Corticosteroids can suppress inflammatory processes and induce maturational effects in different tissues. Maternally administered corticosteroids are used routinely to manage women with premature labor. This treatment enhances fetal lung maturation, and reduces the incidence of intraventricular hemorrhage in premature infants [32]. Antenatal corticosteroids also may accelerate microvascular maturation, because we have shown that maternal corticosteroid administration decreases BBB permeability in preterm ovine fetuses [38-40].
In vitro corticosteroids decrease endothelial cell monolayer permeability and increase transendothelial resistance [22], decrease paracellular permeability and increase TJ protein expression in Schlemm's canal endothelial cells [42], and increase ZO-1 and occludin expression in cerebral endothelial cells [15,36]. Nonetheless, information regarding the in vivo regulation of the constituents of TJs by corticosteroids particularly in fetal subjects is sparse.
Ischemia-reperfusion injury remains a major cause of perinatal brain damage [44]. The constituents of the BBB TJs may help to modulate the homeostasis of the central nervous system in response to injury [21]. Hypoxic-reoxygenation and ischemic-reperfusion injury can alter the permeability and structure of the BBB [16,30,45]. Post-ischemic inflammatory cascades and counteracting repair processes can result in further modification of the BBB [8]. The integrity and potency of TJ proteins in sealing the space between endothelial cells may modulate the response to these pathologic conditions [43].
The effects of antenatal corticosteroids on the response of the fetal BBB to brain ischemia-reperfusion injury have not been examined in-utero. The objective of this study is to test the hypothesis that maternally administered corticosteroids increase the expression of tight junction proteins in the cerebral cortex of premature ovine fetuses with and without exposure to in-utero brain ischemia.
2. Results
The current study presents the results of Western immunoblot analyses of TJ protein expression in the ovine fetal cerebral cortex with and without maternal treatment with corticosteroids, and with and without exposure to in-utero brain ischemia. The frozen cerebral cortical samples for the current immunoblot analyses were performed on samples obtained from fetal sheep used for other purposes in our previous published reports [10,39]. The physiological and hormonal values of the fetuses, and the pathological evaluations of the brains of the fetuses in the current study have been reported [10,39] and will be briefly summarized here. The baseline physiologic variables including the pH, blood gases, hematocrit, heart rate, mean arterial blood pressure values and cortisol concentrations were similar between the placebo/control and dexamethasone/control groups [39]. The baseline physiologic variables also were similar between the fetuses in the placebo/ischemic and dexamethasone/ischemic groups [10]. After ischemia, the physiologic variables remained similar to the baseline values with an exception of minor decreases in the heart rate and increases in plasma cortisol concentrations in the dexamethasone/ischemic group [10]. During ischemia, the number of fetuses with isoelectric EcoG's was similar between the placebo/ischemic and dexamethasone/ischemic groups and carotid blood flow immediately approached zero in the both groups of fetuses and remained in this range during ischemia [10].
As we previously reported, two pathologists who were not aware of the treatment groups [10] scored the coronal brain sections for white matter and cerebral cortical lesions. The most severely damaged area of white matter and cerebral cortex from each section then was selected and separately scored with a grading system (0−5): 0=0%, 1=1−10%, 2=11−50%, 3=51−90%, 4=91−99%, 5=100% of the damaged area. This scoring system was identical to the scoring system that we used in an earlier study [33]. The pathological evaluation of the brains of the fetuses in the placebo/ischemic and dexamethasone/ischemic groups showed that white matter (WM) and cerebral cortical scores did not differ between the groups (WM: 3.0±1.9 and 2.9±1.7; cortex: 3.1±1.7 and 2.6±1.8, mean±S.D.) [10]. The weight of the brains in the dexamethasone/ischemic (39.6±7.0 g) did not differ from those of the placebo/ischemic group (43.3±9.6 g). Brain weights were not available from the fetuses in the placebo/control dexamethasone/control groups.
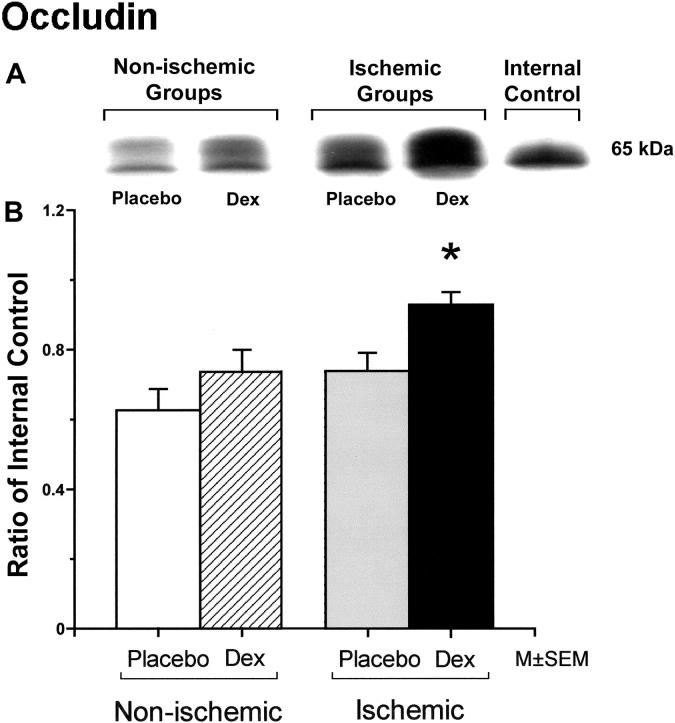
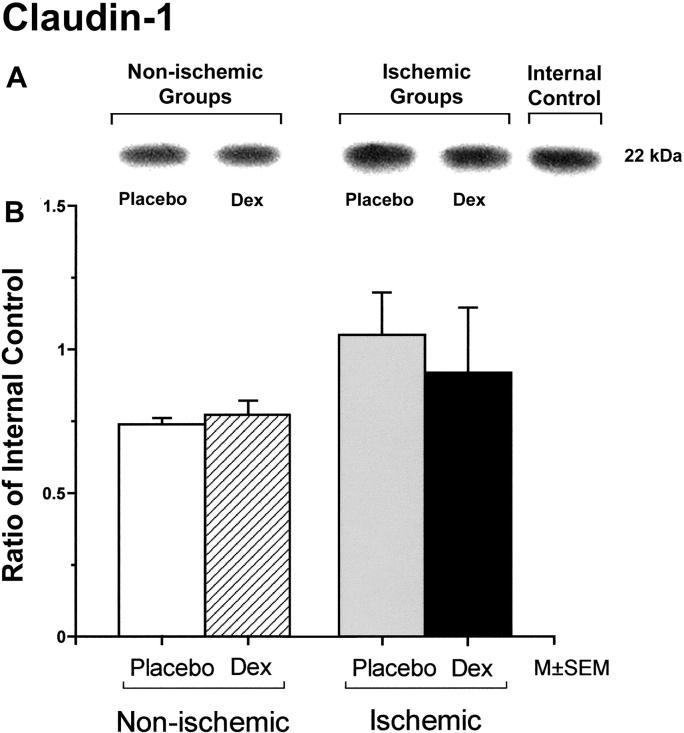
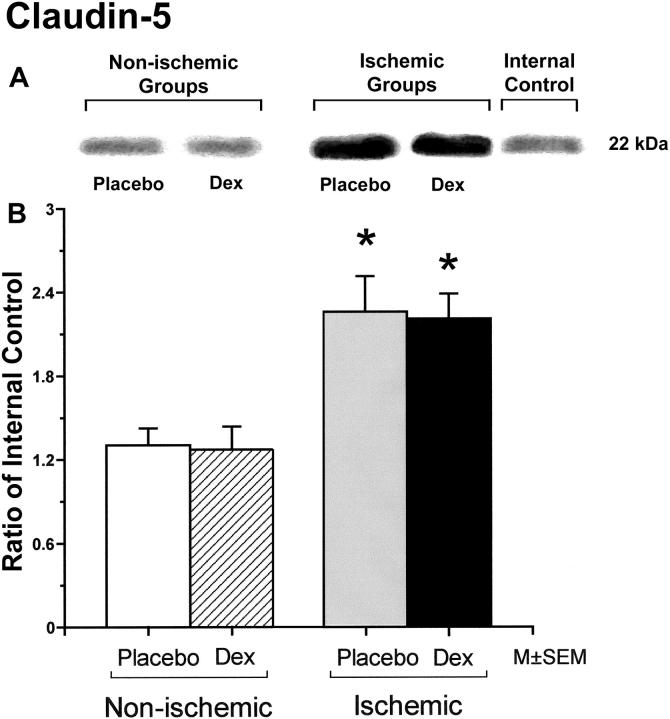
Occludin protein expression was significantly higher in fetuses of the dexamethasone/ischemic than those of the placebo/control group (Figure 1). There were no statistically significant differences in occludin protein expression between the fetuses in the placebo/control and dexamethasone/control or the placebo/control and the placebo/ischemic groups. Claudin-1 protein expression did not differ significantly among the groups (Figure 2). Claudin-5 expression was significantly higher in the fetuses of the placebo/ischemic and dexamethasone/ischemic groups than those in the placebo/control group (Figure 3). There were no significant differences in claudin-5 protein expression between the placebo/control and dexamethasone/control or the placebo/ischemic and dexamethasone/ischemic groups.
Figure 1.
Panel A shows a representative Western immunoblot of occludin protein expression in the cerebral cortex of the fetuses of placebo (Placebo) and dexamethasone (Dex) treated ewes in the non-ischemic groups on the left, and ischemic groups on the right. An internal control sample from the adult brain pool is also shown. Densitometry values from each animal represent the combined densitometry values of both bands of the phosphorylated and non-phosphorylated isoforms of the occludin protein added together. Panel B shows the occludin protein expression in the cerebral cortex plotted as a ratio of the densitometry values in fetuses of the placebo and dexamethasone treated ewes from non-ischemic and ischemic groups to the internal control samples. The open bars represent fetuses of the placebo/control group (n=7), the hatched bar the dexamethasone/control group (n=8), the gray bar the placebo/ischemic (n=5) and the solid bar the dexamethasone/ischemic group (n=5). *P<0.05 versus the to placebo/control group.
Figure 2.
Representative Western immunoblot and bar graph of claudin-1 protein expression in the cerebral cortex of the fetuses of placebo (Placebo) and dexamethasone (Dex) treated ewes in the non-ischemic groups on the left, and ischemic groups on the right. Bars legend as for figure 1. Open bar n=5, hatched bar n=5, gray bar n=6, and solid bar n=5.
Figure 3.
Representative Western immunoblot and bar graph of claudin-5 protein expression in the cerebral cortex of the fetuses of placebo (Placebo) and dexamethasone (Dex) treated ewes in the non-ischemic groups on the left, and ischemic groups on the right. Bars legend as for figure1. Open bar n=5, hatched bar n=6, gray bar n=5 and solid bar n=5. *P<0.05 versus the to placebo/control group.
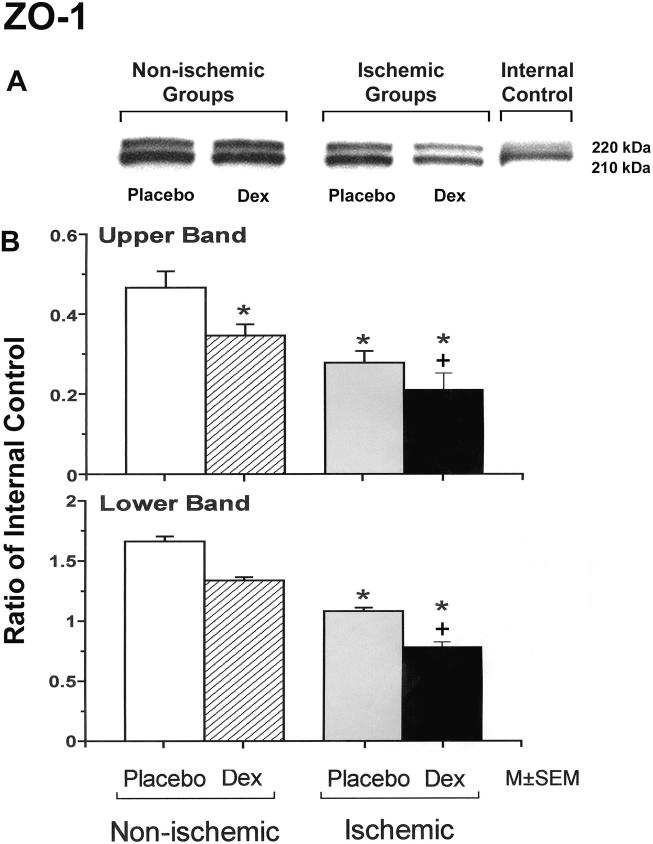
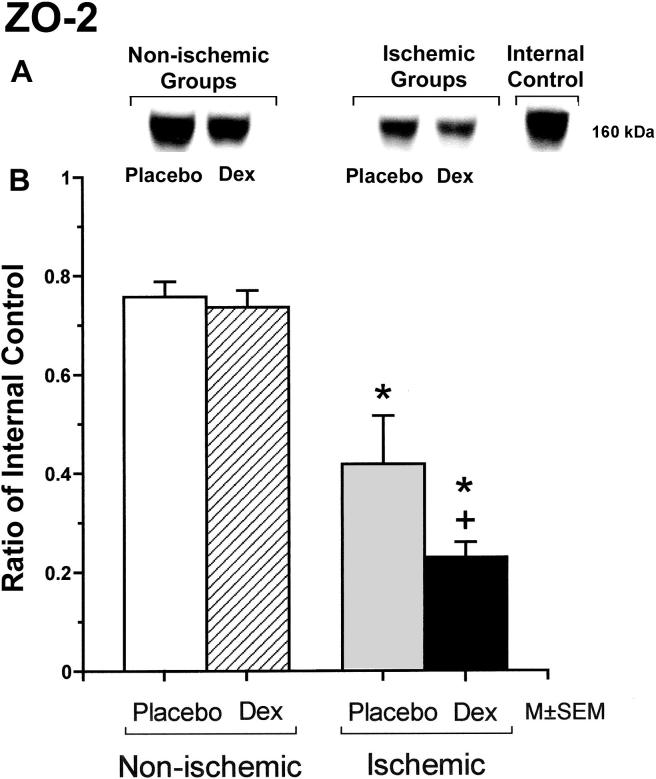
ZO-1 is expressed as two isoforms as a result of alternative RNA splicing differing in the presence of an 80-amino acid region referred to as "motif-a": the 235 kDa upper band corresponds to the ZO-1α+ isoform, and the 225 kDa lower band corresponds to the ZO-1α- isoform. The expression patterns are dynamic and cell specific during development [24,26]. The upper protein band of the ZO-1 expression was significantly lower in the fetuses of the dexamethasone/control, placebo/ischemic and dexamethasone/ischemic groups than in those of the placebo/control group, and in the fetuses of the dexamethasone/ischemic than those of the placebo/ischemic group (Figure 4). The lower protein band of the ZO-1 expression was significantly lower in the fetuses of the placebo/ischemic and dexamethasone/ischemic groups than in those of the placebo/control group, and in the fetuses of the dexamethasone/ischemic than in those of the placebo/ischemic group. ZO-2 protein expression was significantly lower in the fetuses of the placebo/ischemic and dexamethasone/ischemic groups than in those of the placebo/control group, and in the fetuses of the dexamethasone/ischemic than in those of the placebo/ischemic group (Figure 5).
Figure 4.
Representative Western immunoblots and bar graphs of the upper and lower protein bands of ZO-1 expression in the cerebral cortex of the fetuses of placebo (Placebo) and dexamethasone (Dex) treated ewes in the non-ischemic groups on the left, and ischemic groups on the right. ZO-1 is expressed as two isoforms as a result of alternative RNA splicing differing in the presence of an 80-amino acid region referred to as "motif-a": the 235 kDa upper band corresponds to the ZO-1α+ isoform, and the 225 kDa lower band corresponds to the ZO-1α- isoform. The expression patterns are dynamic and cell specific during development [24,26]. Bar legends as for figure 1. Open bars n=6, hatched bars n=6, gray bars n=6 and solid bars n=5 for the upper band and n=6 for the lower band. There were no statistical differences in upper/lower band ratios among the experimental groups (Data not shown).*P<0.05 versus the to placebo/control group. +P<0.05 versus the placebo/ischemic group.
Figure 5.
Representative Western immunoblot and bar graph of ZO-2 protein expression in the cerebral cortex of the fetuses of placebo (Placebo) and dexamethasone (Dex) treated ewes in the non-ischemic groups on the left, and ischemic groups on the right. Bars legends as for figure 1. Open bar n=6, hatched bar n=6, gray bar n=6 and solid bar n=6. *P<0.05 versus the to placebo/control group.+P<0.05 versus the placebo/ischemic group.
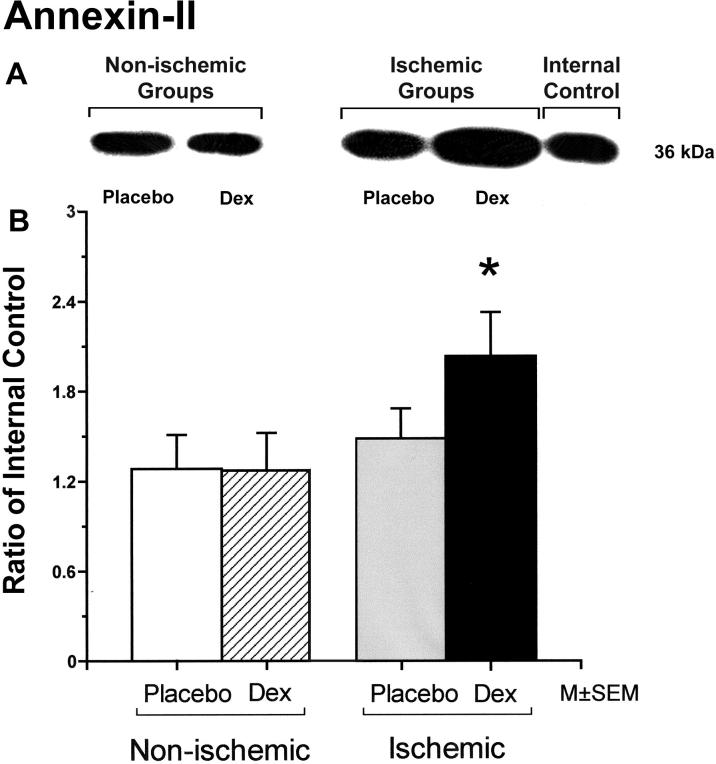
Annexin-II protein expression was significantly higher in fetuses of the dexamethasone/ischemic than those of the placebo/control group (Figure 6). There were no statistically significant differences in annexin-II protein expression between the fetuses in the placebo/control and dexamethasone/control or the placebo/control and the placebo/ischemic groups.
Figure 6.
Representative Western immunoblot and bar graph of annexin-II protein expression in the cerebral cortex of the fetuses of placebo (Placebo) and dexamethasone (Dex) treated ewes in the non-ischemic groups on the left, and ischemic groups on the right. Bars legends as for figure1. Open bar n=5, hatched bar n=6, gray bar n=6 and solid bar n=5. *P<0.05 versus the to placebo/control group.
3. Discussion
Maternally administered corticosteroids have been shown to reduce the incidence of intraventricular hemorrhage in premature infants [29] and to decrease the permeability of the BBB in premature ovine fetuses [39,40]. Corticosteroids have been reported to increase TJ protein expression in Schlemm's canal endothelial cells in-vitro [42], and to increase ZO-1 and occludin expression in cerebral endothelial cells in-vitro [15,36]. Although the molecular structure of the TJ complexes has been elucidated, there is very little information regarding pharmacological regulation of these complexes and/or ischemia related alterations in their molecular composition particularly in vivo and in fetal subjects [1]. Potential changes in the composition of TJ proteins in cerebral vascular endothelial cells could represent one of the mechanism(s) by which maternally administered antenatal corticosteroids result in the decreases in BBB permeability in the fetus [39,40] and reduce the incidence of intraventricular hemorrhage in premature infants [29]. The purpose of the present study was to determine the effects of maternal corticosteroid administration on the molecular composition of the tight junctional complexes in the cerebral cortex of ovine fetuses with and without exposure to in-utero brain ischemia.
The major findings of our study were that: (1) maternal dexamethasone treatment resulted in decreases in fetal cerebral cortical ZO-1 expression, but not in changes in oocludin, claudin-1, claudin-5, ZO-2 or annexin-II expression; (2) ischemia and reperfusion for 72 hours resulted in increases in claudin 5, decreases in ZO-1 and decreases in ZO-2 protein expression; and (3) ischemia-reperfusion after maternal dexamethasone treatment resulted in increases in occludin and annexin-II protein expression, increases in claudin-5 expression that were similar to those observed after ischemia without maternal dexamethasone, and decreases in ZO-1 and ZO-2 protein expression, which were greater than those observed after ischemia-reperfusion without maternal dexamethasone treatment. Taken together our findings can be interpreted to suggest that maternal corticosteroid administration modifies the response of the tight junctional complex to the ischemia-reperfusion cascade, and differentially regulates the composition of proteins expressed in vivo in the cerebral cortex of fetal sheep with and without exposure to brain ischemia and reperfusion.
Carotid artery occlusion with reperfusion was used to induce comparable amounts of injury to the fetal cerebral cortex in the groups exposed to ischemia [17]. We achieved near complete cessation of cerebral blood flow for 30 minutes documented by carotid blood flow measured with flow probes placed around the carotid arteries, and also documented simultaneous isoelectric electrocorticograms in the ischemic fetuses [10]. These conditions resulted in reproducible amounts of brain damage in the fetus [10,17,33]. In the current study, we measured the protein composition of the TJ complexes by Western immunoblot analysis of equal amounts protein extracted from comparable cerebral cortical regions and referenced the experimental samples to the same internal control standard. Although we identified significant differences in the protein composition of the TJ complexes between the placebo/ischemic and dexamethasone/ischemic groups in the current report, we did not observe differences in the amount of ischemic brain damage between these groups on histopathological analysis in our former report [10].
Previous work has shown that hypoxia and ischemia result in alterations in BBB permeability, and in the expression, phosphorylation, and localization of various TJ proteins at the BBB [5,34,45]. Mechanisms underlying these changes potentially include: increases in oxygen free radicals, hypoxia-inducible factor, vascular endothelial growth factor, nitric oxide, and activation of phosphokinases, nuclear factor kappa B, adenylate cyclase and intracellular calcium pathways, as well as direct effects of oxygen and metabolite deprivation, cessation of shear blood flow with resultant invasion of the neurovascular unit by static leukocytes, and the induction of local inflammatory cascades [6,9,12-14,46]. We cannot discern which of these factors could have contributed to the changes in the composition of the TJ proteins that we observed, because we did not measure them in our in vivo fetal sheep studies. However, investigation into mechanism(s), which could have mediated the changes in the TJ protein expression in our study, and the potential mechanism(s) by which dexamethasone potentially modulates such changes, would be of great interest and warrants further investigation.
A number of in vivo BBB permeability studies after ischemia-reperfusion in adult and newborn subjects reported biphasic temporal patterns of barrier reopening during reperfusion [2,8,16,18,20,25,28,30]. An initial early phase occurs during the first few hours, which includes vasogenic cerebral edema and disruption of the BBB resulting in increases permeability to solutes and markers such as Evans Blue. Potential mechanisms include reactive tissue hyperperfusion after ischemia, induction of inflammatory cascades, activation of matrix metalloproteases, and digestion of the BBB extracellular matrix with weakening of the BBB, alterations in transport mechanisms across the BBB such as transcytosis, transporter recruitment, and acute changes in the phosphorylation state and localization of tight junctional proteins. During a second phase, BBB permeability decreases over the next 24−48 hours toward the baseline values. Next in a late third phase, the BBB reopens with a second peak of increased permeability. The mechanisms that underlie this phase may include ongoing activation of inflammatory cascades and secretion of substances such as CCL-2 and other molecules by endothelial cells, astrocytes, and other cells that could alter BBB permeability, as well as, changes in TJ protein expression and localization. After this third phase, repair mechanisms by constituent cells of the BBB may facilitate restitution of BBB function closer to homeostatic conditions. Studies of in vivo models of the BBB have demonstrated alterations in the expression of constituent proteins in patterns that suggest restitution of the vasculature during reperfusion after ischemia [19]. The changes in the composition the TJ proteins that we observed most likely reflect the later phases when the endothelial cell machinery has been activated to increase the production of constitutive proteins to potentially repair BBB damage caused by ischemia-reperfusion. The observed increase in annexin II expression along with occludin supports this contention particularly because annexins appear to enhance the fusion of the lipid bilayer in cells [27]. Whether other cytoplasmic TJ proteins are concomitantly up regulated to match the increases in the membrane bound TJ proteins and, consequently, compensate for the observed decreases in the ZO-1 and ZO-2 expression cannot be determined by our study.
We have previously shown that endogenous increases in plasma cortisol concentrations are associated with ontogenic decreases in barrier permeability during gestation, and that exogenous antenatal corticosteroids decrease BBB permeability at 60% and 80% of gestation in ovine fetuses [38-40]. Several in vitro and in vivo studies have demonstrated structural and functional changes in the TJs of endothelial cells after corticosteroid exposure, which correlated with decreased permeability across the cell layers [7,11,15,22,42]. Corticosteroids also could modulate several of the processes involved in the pathophysiology of BBB disruption during ischemia-reperfusion. Betz and Coester [3] reported decreases in BBB permeability four hours after focal cerebral ischemia in dexamethasone pretreated rats compared with placebo treated rats. Our findings in the ovine fetus support the contention that maternal treatment with corticosteroids modifies the response of the proteins in the tight junctional complex to ischemia-reperfusion.
There are several limitations to our study. The dexamethasone/control and dexamethasone/ischemic groups were not compared because the dose of dexamethasone differed between the groups. However, we did compare the experimental groups with the placebo/control group because the treatment of the ewes was similar, the number of injections and volume of placebo were the same as the volume of dexamethasone or placebo given to the dexamethasone/control, placebo/ischemic and dexamethasone/ischemic groups. The brains of fetuses exposed to ischemia were examined 88 hours after the last of four injections of placebo or dexamethasone, whereas the brains of the fetuses that were not exposed to ischemia were examined 18 hours after the last of four injections of placebo or dexamethasone. Nonetheless, changes in the protein expression between the placebo/control and the experimental groups are not likely to result from the small differences in the gestational ages, i.e. 2−4 days, or differences in the timing of the placebo injections before the samples were obtained. Although examination of the fetal sheep brain at different intervals after ischemia also would have been of great interest, we were not able to study a sufficient number of fetuses in this large animal model to examine different intervals of reperfusion.
There appears to be differential regulation of the expression of the different proteins constituting the TJ complex after dexamethasone treatment with and without exposure to brain ischemia and reperfusion. The membrane associated TJ proteins such as occludin and claudins are up regulated, whereas some cytosolic TJ proteins such as ZO-1 and ZO-2 are down regulated. Whether other cytoplasmic TJ proteins are concomitantly up regulated to match the increases in the membrane bound TJ proteins and, consequently, compensate for the observed decreases in the ZO-1 and ZO-2 expression cannot be discerned by our study. We also have not presented immunohistochemical data on the tight junctional complexes, as we did not have cerebral cortical tissue appropriately preserved for this purpose from our previous work [10,39] and we did not measure BBB permeability in the animals exposed to brain ischemia. In the absence of this additional data, the functional significance of the differential changes in the composition of the TJ proteins cannot be elucidated and warrants further investigation. In spite of the potential limitations of our study, we observed significant changes in protein expression among the experimental groups ranging from 48% to 70%.
In summary, our study was the first to examine the expression of proteins in the tight junctional complex after cerebral ischemia-reperfusion in the fetus. Our findings suggest increases and decreases in certain key proteins in the TJ complex in the fetal cerebral cortex after maternal treatment with antenatal corticosteroids and ischemiareperfusion injury. We conclude that maternal corticosteroid treatment differentially regulates the expression of component proteins of the TJs in the cerebral cortex of ovine fetuses exposed to brain ischemia. The functional significance of this differential regulation of TJ protein expression warrants further investigation.
4. Experimental Procedure
This study was conducted after approval by the Institutional Animal Care and Use Committees of Brown University and Women and Infants' Hospital of Rhode Island and according to the National Institutes of Health Guidelines for use of experimental animals.
Study groups.
The twenty-five fetuses of mixed breed ewes were examined from one of four study groups. Seven non-ischemic fetuses of placebo treated ewes were assigned to a placebo/control group; eight non-ischemic fetuses of dexamethasone treated ewes were assigned to a dexamethasone/control group; five ischemic fetuses of placebo treated ewes were assigned to a placebo/ischemic group; and five ischemic fetuses of dexamethasone treated ewes were assigned to a dexamethasone/ischemic group. The tissue samples for this study were obtained from animals in our previous reports examining the effects of antenatal corticosteroids on BBB permeability [39], and the effects of antenatal corticosteroids upon pathological ischemic brain injury the ovine fetus [10].
Animal preparation.
The details of the surgical preparation for the study groups summarized above have been previously reported [10,39]. Briefly, surgery was performed for the placebo/control and dexamethasone/control groups under halothane anesthesia on 15 mixed breed ewes at 112−113 days of gestation as previously described [39]. Full term gestation is 150 days. Catheters were placed in the fetuses in the ascending aorta for blood samples, descending aorta for heart rate and blood pressure and amniotic fluid for pressure monitoring, and in ewes into a femoral artery [39]. In fetuses in the placebo/ischemic and dexamethasone/ischemic groups, surgery was performed as described above at 112−117 days of gestation. In addition, bilateral inflatable occluders (In Vivo Metric, Healdsburg, CA) and flow probes (Transonic Systems, Ithaca, NY) were placed around the carotid arteries [10]. Two pairs of screws (Small Parts, Miami Lakes, FL) also were placed onto the dura and connected to a recorder by insulated wires (Alpha Wire, Elizabeth, NJ) to measure the electrocorticogram (EcoG) [10]. The EcoG was measured to determine if the fetal cerebral cortical brain wave pattern became isoelectric during ischemia [10].
Experimental protocol and methodology.
Two-four days after recovery from surgery in the non-ischemic placebo/control and dexamethasone/control groups, the ewes were given either four 6 mg dexamethasone (4 mg/ml, Fujisawa, USA, Deerfield, IL, n=8) or placebo (1.5 ml of 0.9% NaCl, n=7) by intramuscular injections every 12 hours for 48 hours. The dose of dexamethasone used in these studies was based upon recommendations for fetal maturation in pregnant women with premature labor [31]. Dexamethasone was used because it is one of the most extensively used corticosteroids for accelerating fetal maturation, has been widely used in studies of the central nervous system (CNS), and is also used to treat CNS disorders [40]. The final injection of placebo or dexamethasone was given 18 hours before the tissue samples were obtained at 117−120 days of gestation. The ewe was given intravenous pentobarbital (15−20 mg/kg) to achieve a surgical plane of anesthesia. A hysterotomy was performed, the fetus was withdrawn from the uterus, and the brain removed for the tissue samples. Then, the ewe was killed with pentobarbital (100−200 mg•kg−1). A portion of the frontal cerebral cortex was snap frozen and stored at −80° until analysis.
The ewes in the placebo/ischemic and dexamethasone/ischemic groups were given either four 4 mg dexamethasone (Fujisawa, USA, Deerfield, IL n=5) or placebo (0.9% NaCl, n=5) by intramuscular injections every 12 hours for 48 hours. The final injections in these groups were given 12 hours before the onset of the studies at 120−124 days of gestation. Although we used the 6 mg dexamethasone dose as described above in the non-ischemic groups, we used a 4 mg dose in the ischemic groups, because we found that fetuses of ewes exposed to the 6 mg dose, surgery and brain ischemia aborted [10]. Therefore, it was not possible to treat the ewes with the 6 mg dose in the dexamethasone/ischemic group [10]. Briefly, in the placebo/ischemic and dexamethasone/ischemic groups, after baseline physiologic measurements were obtained, brain ischemia was induced for 30 min by inflating the carotid occluders with 0.9% NaCl as previously described [10]. The 30-minute interval of ischemia was based upon studies demonstrating that this was optimal for the development of brain ischemia, and that neuronal rescue remained possible after this duration of occlusion [17,35]. During the bilateral carotid artery occlusion, the EcoG and carotid blood flow were recorded [10]. Then, the occluders were deflated and reperfusion continued for 72 hours. Measurements for the previous study were repeated at 0.25, 1, 2, 24, 48, and 72 hours of reperfusion [10]. The ewe was treated as above and the frontal cerebral cortex was snap frozen as above.
Western immunoblot protein detection and quantification.
The abundance of occludin, claudin-1, claudin-5, ZO-1, ZO-2, and annexin-II were measured by Western immunoblot. Frontal cerebral cortical samples were homogenized in Triton/Deoxycholate/SDS (100mM NaCl, 1% Triton X, 0.5 Sodium Deoxycholate, 0.2% SDS, 2 mM EDTA, 1 mM benzamidine) buffer containing 1% protease inhibitor cocktail (Sigma, Saint Louis, Missouri) and 1% Phenylmethylsulfonyl Fluoride (PMSF, Calbiochem, San Diego, CA) solution on ice for 10 minutes, and centrifuged at 1.4 × 104 rpm for 30 minutes to extract the membrane-associated proteins i.e. occludin, claudin-1, claudin-5 and annexin-II. Urea buffer (6 M urea, 150 mM NaCl, 5mM MgCl2, 5 mM EGTA, 10 mM Tris, pH 8.0, 1% TX-100) was used to extract intra-cytoplasmic proteins i.e. ZO-1 and ZO-2. The total protein concentration of the supernatant was determined by a bicinchoninic acid protein assay (BCA-200 protein assay kit, Pierce, Rockford, IL).
Aliquots adjusted for equal loading of 50 μg of protein in 50 μl of solution were loaded on SDS-polyacrylamide gel and immunoblotted to PVDF membranes (polyvinylidene diflouride, 0.2 micron, Bio-Rad Laboratories, Hercules, CA) using a semi-dry technique. Ten percent polyacrylamide gels were use for occludin, 16% for claudin-1 and claudin-5 and seven percent for ZO-1 and ZO-2 and 12 % for annexin II. The membranes were blocked with ten percent non-fat milk in TBST (Tris-buffered saline with Tween) buffer for one hour at room temperature washed in TBST three times for ten minutes per wash, and incubated overnight at 4°C with the appropriate primary antibody solution. The proteins were probed with the following primary antibodies: occludin with polyclonal rabbit anti-human (Zymed, South San Francisco, Calif.) at a dilution of 1:2500; claudin-1 with polyclonal rabbit anti-human (Zymed) at a dilution of 1:6000, ZO-2 with polyclonal rabbit anti-human (Zymed) at a dilution of 1:5000; and claudin-5 with monoclonal mouse anti-human (Zymed) at a dilution of 1:5000, ZO-1 with monoclonal mouse anti-human (Zymed) at a dilution 1:5000, and annexin-II with monoclonal mouse anti-human (Zymed) at a dilution of 1:5000. Detection of the band was dependent on incubation with primary antibody, omission of which resulted in the absence of the chemiluminescent signal.
The immunoblots were washed in TBST three times for ten minutes per wash and then incubated for one hour at room temperature with goat anti-rabbit secondary antibodies (Alpha Diagnostic, San Antonio, Texas) at dilution of 1:5,000 for occludin and 1:10,000 for claudin-1 and ZO-2, and goat anti-mouse secondary antibodies (Zymed) at a dilution of 1:10,000 for claudin-5, ZO-1 and annexin II. The immunoblots were again washed four times in TBST for ten minutes per wash. Binding of the secondary antibody was detected with enhanced chemiluminescence (ECL-plus) Western blotting detection reagents (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) before exposure to Hyperfilm ECL (Amersham Pharmacia Biotech, Inc., Piscataway, NJ).
All experimental samples were normalized to a reference protein standard that had been obtained from a homogenate pool from the cerebral cortex of one adult sheep. As we have previously described, these samples served as internal control reference standards for quality control for loading, transfer, verification of potential internal variability across the gel, and for normalization of the cerebral cortical densitometric values to permit accurate comparisons against a single internal control among the different immunoblots and groups [23]. For the purpose of this report, we refer to the internal control reference standards from the adult sheep cerebral cortex hereafter as the internal control samples.
Each immunoblot included four series of samples with each series containing one sample from each experimental group and one internal control sample. We calculated a coefficient of variation for the internal control samples on each immunoblot. The values for the experimental samples in this study were accepted as valid only if the percent coefficient of variation for the internal control samples on each immunoblot was less than 20%. The final values represented an average of the densitometry values obtained from the different immunoblots. We have previously shown that this method correlates well with values that have been normalized as ratios to β-actin [23]. We also have used a similar normalization technique to examine the ontogeny and effects of corticosteroid pretreatment on aquaporin water channels in the ovine cerebral cortex [37]. Uniformity in inter-lane loading was also established by Coomassie blue (Sigma, St. Louis, MO) staining of the polyacrylamide gels, and uniformity of transfer to the PVDF membranes confirmed by Ponceau S (Sigma, St. Louis, MO) [41].
MDCK (BD Biosciences, San Diego, CA) was used as a positive control for occludin, claudin-1, ZO-1 and ZO-2 and rat lung for claudin-5 and annexin II. Western immunoblots preformed without the primary antibodies were used to rule out non-specific binding. Detection of the occludin, claudin-1, claudin-5, ZO-1, ZO-2 and annexin II bands at 22, 22, 220 and 210 (ZO-1), 160 and 36 kDa, respectively, were dependent on incubation with primary antibody, omission of which resulted in the absence of this signal.
Densitometric analysis.
Hyperflilm ECL (Amersham Pharmacia Biotech, Inc.) film was scanned and the densitometries of the bands measured with Gel-Pro Analyzer software (Media Cybernetics, Silver Spring, MD). The final values represented an average of the normalized values of each experimental sample on different immunoblots.
Statistical analysis.
Two-way analysis of variance (ANOVA) was used to compare the values among the fetuses in the placebo/control, dexamethasone/control, placebo/ischemic and dexamethasone/ischemic groups (Statistica Software, Stat Soft Inc., Tulsa, OK). If a significant difference was found by ANOVA, the Fisher least significant difference test was used to detect specific differences among groups. The dexamethasone/control and dexamethasone/ischemic groups were not compared statistically because the dose of dexamethasone differed between the groups. However, the placebo/control group was compared with the dexamethasone/control, placebo/ischemic, and dexamethasone/ischemic groups because the treatment of the ewes was similar, the number of injections and the volume of 0.9% NaCl were the same as the volume of dexamethasone administered to both the dexamethasone/control and dexamethasone/ischemic groups. Values are expressed as mean±SEM. Differences are considered statistically significant at P<0.05.
Acknowledgment
Supported by NIH R01-HD34618.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 2.Belayev L, Busto R, Ikeda M, Rubin LL, Kajiwara A, Morgan L, Ginsberg MD. Protection against blood-brain barrier disruption in focal cerebral ischemia by the type IV phosphodiesterase inhibitor BBB022: a quantitative study. Brain Res. 1998;787:277–285. doi: 10.1016/s0006-8993(97)01499-6. [DOI] [PubMed] [Google Scholar]
- 3.Betz AL, Coester HC. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990;21:1199–1204. doi: 10.1161/01.str.21.8.1199. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury MWB. Physiopathology of the blood-brain barrier. Plenum; New York: 1976. pp. 507–516. [DOI] [PubMed] [Google Scholar]
- 5.Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun. 2005;327:1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- 6.Brown RC, Mark KS, Egleton RD, Davis TP. Protection against hypoxia-induced blood-brain barrier disruption: changes in intracellular calcium. Am J Physiol Cell Physiol. 2004;286:C1045–1052. doi: 10.1152/ajpcell.00360.2003. [DOI] [PubMed] [Google Scholar]
- 7.Cucullo L, Hallene K, Dini G, Dal Toso R, Janigro D. Glycerophosphoinositol and dexamethasone improve transendothelial electrical resistance in an in vitro study of the blood-brain barrier. Brain Res. 2004;997:147–151. doi: 10.1016/j.brainres.2003.09.079. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2006;26:797–810. doi: 10.1038/sj.jcbfm.9600229. [DOI] [PubMed] [Google Scholar]
- 9.Dux E, Temesvari P, Joo F, Adam G, Clementi F, Dux L, Hideg J, Hossmann KA. The blood-brain barrier in hypoxia: ultrastructural aspects and adenylate cyclase activity of brain capillaries. Neuroscience. 1984;12:951–958. doi: 10.1016/0306-4522(84)90182-9. [DOI] [PubMed] [Google Scholar]
- 10.Elitt CM, Sadowska GB, Stopa EG, Pinar H, Petersson KH, Stonestreet BS. Effects of antenatal steroids on ischemic brain injury in near-term ovine fetuses. Early Hum Dev. 2003;73:1–15. doi: 10.1016/s0378-3782(03)00030-6. [DOI] [PubMed] [Google Scholar]
- 11.Felinski EA, Antonetti DA. Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Curr Eye Res. 2005;30:949–957. doi: 10.1080/02713680500263598. [DOI] [PubMed] [Google Scholar]
- 12.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276:C812–820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 13.Fischer S, Wiesnet M, Marti HH, Renz D, Schaper W. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J Cell Physiol. 2004;198:359–369. doi: 10.1002/jcp.10417. [DOI] [PubMed] [Google Scholar]
- 14.Fleegal MA, Hom S, Borg LK, Davis TP. Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol. 2005;289:H2012–2019. doi: 10.1152/ajpheart.00495.2005. [DOI] [PubMed] [Google Scholar]
- 15.Forster C, Silwedel C, Golenhofen N, Burek M, Kietz S, Mankertz J, Drenckhahn D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol. 2005;565:475–486. doi: 10.1113/jphysiol.2005.084038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- 17.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hai J, Lin Q, Li ST, Pan QG. Chronic cerebral hypoperfusion and reperfusion injury of restoration of normal perfusion pressure contributes to the neuropathological changes in rat brain. Brain Res Mol Brain Res. 2004;126:137–145. doi: 10.1016/j.molbrainres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Haqqani AS, Nesic M, Preston E, Baumann E, Kelly J, Stanimirovic D. Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLCMS/MS. Faseb J. 2005;19:1809–1821. doi: 10.1096/fj.05-3793com. [DOI] [PubMed] [Google Scholar]
- 20.Harris NG, Gauden V, Fraser PA, Williams SR, Parker GJ. MRI measurement of blood-brain barrier permeability following spontaneous reperfusion in the starch microsphere model of ischemia. Magn Reson Imaging. 2002;20:221–230. doi: 10.1016/s0730-725x(02)00498-8. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 22.Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, Galla HJ. Hydrocortisone reinforces the blood-brain properties in a serum free cell culture system. Biochem Biophys Res Commun. 1998;247:312–315. [PubMed] [Google Scholar]
- 23.Kim CR, Sadowska GB, Petersson KH, Merino M, Sysyn GD, Padbury JF, Stonestreet BS. Effects of postnatal steroids on Na+/K+-ATPase activity and alpha1- and beta1-subunit protein expression in the cerebral cortex and renal cortex of newborn lambs. Reprod Fertil Dev. 2006;18:413–423. doi: 10.1071/rd05114. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Renihan MK, Boulianne GL. Characterization of big bang, a novel gene encoding for PDZ domain-containing proteins that are dynamically expressed throughout Drosophila development. Gene Expr Patterns. 2006;6:504–518. doi: 10.1016/j.modgep.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Krizanac-Bengez L, Mayberg MR, Cunningham E, Hossain M, Ponnampalam S, Parkinson FE, Janigro D. Loss of shear stress induces leukocyte-mediated cytokine release and blood-brain barrier failure in dynamic in vitro blood-brain barrier model. J Cell Physiol. 2006;206:68–77. doi: 10.1002/jcp.20429. [DOI] [PubMed] [Google Scholar]
- 26.Kurihara H, Anderson JM, Farquhar MG. Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci U S A. 1992;89:7075–7079. doi: 10.1073/pnas.89.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DB, Jamgotchian N, Allen SG, Kan FW, Hale IL. Annexin A2 heterotetramer: role in tight junction assembly. Am J Physiol Renal Physiol. 2004;287:F481–491. doi: 10.1152/ajprenal.00175.2003. [DOI] [PubMed] [Google Scholar]
- 28.Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, Yang SH. 17beta-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060:55–61. doi: 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 29.Ment LR, Oh W, Ehrenkranz RA, Philip AG, Duncan CC, Makuch RW. Antenatal steroids, delivery mode, and intraventricular hemorrhage in preterm infants. Am J Obstet Gynecol. 1995;172:795–800. doi: 10.1016/0002-9378(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 30.Mirro R, Armstead WM, Busija DW, Leffler CW. Blood to brain transport after newborn cerebral ischemia/reperfusion injury. Proc Soc Exp Biol Med. 1991;197:268–272. doi: 10.3181/00379727-197-43254. [DOI] [PubMed] [Google Scholar]
- 31.National Institutes of Health Consensus Development Panel Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 32.Newnham JP, Moss TJ, Nitsos I, Sloboda DM. Antenatal corticosteroids: the good, the bad and the unknown. Curr Opin Obstet Gynecol. 2002;14:607–612. doi: 10.1097/00001703-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Petersson KH, Pinar H, Stopa EG, Faris RA, Sadowska GB, Hanumara RC, Stonestreet BS. White matter injury after cerebral ischemia in ovine fetuses. Pediatr Res. 2002;51:768–776. doi: 10.1203/00006450-200206000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Plateel M, Teissier E, Cecchelli R. Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neurochem. 1997;68:874–877. doi: 10.1046/j.1471-4159.1997.68020874.x. [DOI] [PubMed] [Google Scholar]
- 35.Raad RA, Tan WK, Bennet L, Gunn AJ, Davis SL, Gluckman PD, Johnston BM, Williams CE. Role of the cerebrovascular and metabolic responses in the delayed phases of injury after transient cerebral ischemia in fetal sheep. Stroke. 1999;30:2735–2741. doi: 10.1161/01.str.30.12.2735. [DOI] [PubMed] [Google Scholar]
- 36.Romero IA, Radewicz K, Jubin E, Michel CC, Greenwood J, Couraud PO, Adamson P. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003;344:112–116. doi: 10.1016/s0304-3940(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 37.Ron NP, Kazianis JA, Padbury JF, Brown CM, McGonnigal BG, Sysyn GD, Sadowska GB, Stonestreet BS. Ontogeny and the effects of corticosteroid pretreatment on aquaporin water channels in the ovine cerebral cortex. Reprod Fertil Dev. 2005;17:535–542. doi: 10.1071/rd03044. [DOI] [PubMed] [Google Scholar]
- 38.Stonestreet BS, Patlak CS, Pettigrew KD, Reilly CB, Cserr HF. Ontogeny of blood-brain barrier function in ovine fetuses, lambs, and adults. Am J Physiol. 1996;271:R1594–1601. doi: 10.1152/ajpregu.1996.271.6.R1594. [DOI] [PubMed] [Google Scholar]
- 39.Stonestreet BS, Petersson KH, Sadowska GB, Pettigrew KD, Patlak CS. Antenatal steroids decrease blood-brain barrier permeability in the ovine fetus. Am J Physiol. 1999;276:R283–289. doi: 10.1152/ajpregu.1999.276.2.R283. [DOI] [PubMed] [Google Scholar]
- 40.Stonestreet BS, Sadowska GB, McKnight AJ, Patlak C, Petersson KH. Exogenous and endogenous corticosteroids modulate blood-brain barrier development in the ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2000;279:R468–477. doi: 10.1152/ajpregu.2000.279.2.R468. [DOI] [PubMed] [Google Scholar]
- 41.Tseng YT, Yano N, Rojan A, Stabila JP, McGonnigal BG, Ianus V, Wadhawan R, Padbury JF. Ontogeny of Phosphoinositide 3-Kinase (PI3K) Signaling in Developing Heart: Effect of Acute {beta}-Adrenergic Stimulation. Am J Physiol Heart Circ Physiol. 2005 doi: 10.1152/ajpheart.00435.2005. [DOI] [PubMed] [Google Scholar]
- 42.Underwood JL, Murphy CG, Chen J, Franse-Carman L, Wood I, Epstein DL, Alvarado JA. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am J Physiol. 1999;277:C330–342. doi: 10.1152/ajpcell.1999.277.2.C330. [DOI] [PubMed] [Google Scholar]
- 43.Vaccarino FM, Ment LR. Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed. 2004;89:F190–192. doi: 10.1136/adc.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volpe J. Hypoxic-Ischemic Encephalopathy. Neurology of the Newborn. W.B. Saunders Company; Philadelphia, PA: 2001. pp. 260–313. [Google Scholar]
- 45.Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxiareoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285:H2820–2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- 46.Witt KA, Mark KS, Huber J, Davis TP. Hypoxia-inducible factor and nuclear factor kappa-B activation in blood-brain barrier endothelium under hypoxic/reoxygenation stress. J Neurochem. 2005;92:203–214. doi: 10.1111/j.1471-4159.2004.02871.x. [DOI] [PubMed] [Google Scholar]