Abstract
The induction of proinflammatory cytokines in stressed myocardium is considered an innate immune response, but the role of β-adrenergic signaling in this proinflammatory response and the mechanisms of cardioprotection by β-blockers are not fully understood. In the present study, we analyzed interleukin-6 (IL-6) formation and promoter activation in β-adrenoceptor-stimulated neonatal rat cardiomyocytes, in transgenic mice with cardiac overexpression of β1-adrenoceptors, and in failing human myocardium. IL-6 formation and release in cultured cardiomyocytes under β-adrenoceptor stimulation requires the activation of activating protein-1 (AP-1) binding sites and of cAMP response elements (CRE) in the IL-6 promoter, but this release (140 ± 6 pg/mL medium under 10−6 M isoproterenol vs. 81 ± 3 pg/mL unstimulated, P < 0.05) is moderate compared with that under inflammatory stimulation (855 ± 44 pg/mL, endotoxin 0.1μg/mL). Similarly, IL-6 is induced together with CRE- and AP-1 activation in the left ventricle (LV) of β1-transgenic mice before the onset of failure. However, we observed IL-6 induction with activation of NF-κB in addition to CRE and AP-1 in β1-transgenic mice at the age of 22 weeks and in explanted human LV after full development of failure. Treatment with β-blockers lowered myocardial IL-6 as well as AP-1, NF-κB, and CRE activation. Therefore, the activation of AP-1 and CRE is part of β-adrenergic signal transduction for IL-6 induction in nonfailing and failing cardiomyocytes, whereas NF-κB activation contributes only in overloaded failing myocardium.
INTRODUCTION
Proinflammatory cytokines, including tumor necrosis factor α, interleukin (IL)-1, and IL-6, are barely expressed in the healthy myocardium, but they are substantially induced in cardiomyocytes by any form of cardiac overload or injury. This rather uniform reaction has been considered an “innate immune response of the heart” (1). The acute activation of this proinflammatory stress response initially provides the heart with adaptive and protective mechanisms (2). However, the sustained and strong activation of proinflammatory cytokines in the myocardium converts into maladaption, contributing to overt cardiac decompensation and failure (2).
Important signals for the cardiac induction of proinflammatory cytokines are redox stress, ischemia/reperfusion, myocyte stretch, release of molecules from damaged cells, and the enhanced neuroendocrine activity in chronic heart failure (1). Within the latter, potentiating interactions between an activated renin-angiotensin system and proinflammatory cytokines are well documented (3). The effects of sympathetic overactivity on the proinflammatory stress response are less clear: β-adrenoceptor activation is considered as one contributor to stress-induced immunosuppression (4). However, chronic isoproterenol infusion in rats induces myocardial expression of IL-6 and other cytokines (5), but it is not clear whether this induction occurs directly via cAMP generation or indirectly owing to free radical generation, calcium overload, myocyte hypertrophy, or myocardial ischemia. Primary cardiac fibroblasts (6,7) and cardiomyocytes (8) have been reported to express IL-6 after adrenergic stimulation or myocardial infarction (9). However, β-blockade in murine postinfarction heart failure differentially affects myocardial cytokine expression without lowering IL-6 expression (9,10).
The promoter region of the rat IL-6 gene contains a well characterized cAMP-responsive element (CRE), and binding sites for activating-protein-1 (AP-1), NF-κB, and others (11,12). These promoter elements are conserved among species, such as human, mouse, or rat. We used the binding sites in the promoter of the IL-6 gene to analyze the myocardial expression of this cytokine under various conditions of β-adrenoceptor activation in vitro and in vivo. From data in cardiomyocytes in culture, mouse myocardium with cardiomyocyte-specific β1-adrenoceptor overexpression, and failing human myocardium, we conclude that β-adrenoceptor-mediated activation of CRE and AP-1 directly contributes to IL-6 induction in healthy and failing myocardium.
MATERIALS AND METHODS
Cardiomyocyte Culture
Preparation and cultivation of monolayer cultures of spontaneously contracting neonatal rat cardiomyocytes from hearts of 1–3-day-old Wistar rats was performed as described (13). Muscle cells were separated from nonmuscle cells by means a differential attachment technique. The suspension of these cells in CMRL-1415-ATM medium containing 10% calf serum, 10% horse serum, 10 mM HEPES, and 0.02 mg/mL tobramycin was distributed into 25 cm2 plastic culture flasks or six wells at 1.5 × 105 cells/cm2 for 48 h. After 48 h the serum-supplemented medium was replaced by serum-free CMRL-1415-ATM medium containing 10 mM HEPES, 0.1 μM dexamethasone, 5 μM insulin, 0.4 μM iron-saturated transferrin, 0.02 mg/mL tobramycin, and 10 μM cytosin-1β-D-furanoarabinoside to stop proliferation of non-cardiomyocytes. Neonatal cardiomyocytes were maintained in serum-free medium for 24 h and then exposed to catecholamines as described in the figure legends. Preincubation with β-blockers was started one hour before catecholamine stimulation. Routinely, the viability of attached cardiomyocytes was checked by the trypan blue dye exclusion method. IL-6 immunocytochemistry showed no preferential IL-6 staining of nonmyocytes, identified by α-sarcomeric actin, vimentin, or von Willebrand factor staining, under isoproterenol or norepinephrine (10−6 M).
Animal Model
Mice with transgenic overexpression of the β1 adrenergic receptor under the control of the α-myosin heavy chain promoter and wild-type mice (14) were used to study the effect of chronic β-adrenergic stimulation on IL-6 expression in left ventricle (LV) tissue (n = 8 per group; age 8–22 weeks). It has previously been reported that these transgenic animals show increased contractility compared with wild-type mice at a young age, but this difference is lost at 16 weeks and contractility continues to decrease thereafter (14).
Patients
Explanted tissue specimens from the free wall of the LV from heart failure patients with dilated cardiomyopathy (DCM) or end-stage ischemic cardiomyopathy (ICM) and from 21 donor hearts (from 15 men and 6 women, age 40 ± 4 years) were investigated in this study. Patient characteristics are provided in Table 1. The donor hearts were not used for transplantation for technical reasons but were free of detectable ventricular hypertrophy or major coronary artery sclerosis. All samples analyzed in this study were free of visible fibrosis or necrosis as judged by the explanting cardiac surgeon. The use of human tissue was approved by the ethics committee of the Martin Luther University Halle-Wittenberg.
Table 1.
Patient Characteristics.
| DCM | ICM | |
|---|---|---|
| Number of patients | 30 | 13 |
| Sex (male/female) | 29/1 | 12/1 |
| Age (years) | 52 ± 1 | 51 ± 2 |
| Hemodynamics | ||
| EF (%) | 22 ± 2 | 26 ± 2 |
| Heart rate (min−1) | 88 ± 4 | 81 ± 5 |
| PCWP (mmHg) | 22 ± 2 | 18 ± 2 |
| MAP (mmHg) | 82 ± 2 | 79 ± 2 |
| PAP (mmHg) | 30 ± 2 | 28 ± 3 |
| Cardiac index (L/min/m2) | 2.1 ± 0.1 | 2.5 ± 0,2 |
| Comorbiditya | ||
| Atrial fibrillation (n) | 14 | 7 |
| Hypertension (n) | 9 | 10 |
| Diabetes (n) | 5 | 6 |
| Hyperlipidemia (n) | 6 | 9 |
| Preexplantation treatments | ||
| β-blocker (n) | 15 | 7 |
| Glycoside (n) | 18 | 11 |
| Other antiarrhythmics (n) | 5 | 0 |
| Anticoagulation (n) | 27 | 13 |
| ACE inhibitor/AT1 blocker (n) | 26 | 12 |
| Nitrate (n) | 3 | 13 |
| Lipid-lowering drug (n) | 6 | 9 |
Comorbidities appeared later or were not regarded as causal for the cardiomyopathy by the cardiac surgeon. DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy; EF, ejection fraction; PCWP, pulmonary capillary wedge pressure; MAP, mean systemic arterial pressure; PAP, pulmonary artery pressure.
RNA Extraction
Total RNA was isolated from LV tissue by guanidine thiocyanate/cesium chloride centrifugation and from cardiomyocytes by using TRI-Reagent® (Sigma-Aldrich Chemie, Taufkirchen, Germany). Integrity and quality of the RNA was confirmed by agarose gel electrophoresis, and the concentration was determined by measuring UV-absorption at 260 nm.
RT-PCR
Reverse transcription (RT) of RNA samples was carried out for 30 min at 42°C. RT-PCR was performed by standard protocols; the whole PCR reaction was electrophoresed on a 1% agarose gel. The following primer pairs were used: rat IL-6 sense 5′-CCA CTG CCT TCC CTA CTT CA-3′, rat IL-6 antisense 5′-TGG TCC TTA GCC ACT CCT TCT-3′, human IL-6 sense 5′-CTC AGC CCT GAG AAA GGA GA-3′, human IL-6 antisense 5′-TGC AGG AAC TCC TTA AAG CTG-3′, mouse IL-6 sense 5′-AGT TGC CTT CTT GGG ACT GAT-3′, and mouse IL-6 antisense 5′-GGA AAT TGG GGT AGG AAG GA-3′. The products of expected size were gel-purified and sequenced. Densitometric analysis of the PCR products was performed with the AIDA data acquisition and evaluation software (raytest, Straubenhardt, Germany). Data are expressed as IL-6 mRNA/18S rRNA.
Northern Blotting
Total RNA (20 μg) was size fractioned (1% MOPS/formaldehyde gel), transferred to nylon membrane, fixed by UV crosslinking, and hybridized with a radiolabeled cDNA probe (rat IL-6). Loading was monitored with ethidium bromide staining and by probing with digoxigenin-labeled 18S rRNA.
Western Blotting of IL-6
Frozen LV tissue was rapidly homogenized in a buffer containing 50 mM Tris HCl, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 1% sodiumdeoxycholate, and protease inhibitor cocktail (Sigma-Aldrich Chemie). Proteins were quantified using the BCA Protein Assay (Perbio Science Deutschland, Bonn, Germany); 50 μg of protein was loaded on 10% SDS-PAGE gel. After electrophoresis, proteins were transferred to a nitrocellulose membrane. The filters were blocked and then incubated with antibodies directed against rat IL-6 (1:1000, Biosource, Solingen, Germany) and rat/human/mouse IL-6 (1:1000, Endogen, Bonn, Germany). After incubation with peroxidase-conjugated secondary antibody, blots were subjected to the enhanced chemiluminescent detection method (Amersham, München, Germany) and exposed to a Kodak MR film (Sigma-Aldrich Chemie).
IL-6 ELISA
The release of IL-6 from neonatal rat cardiomyocytes into the medium was quantified by ELISA kit (limit of detection: 15.6 pg/mL) (R&D Systems, Wiesbaden, Germany.
Generation of Mutants of Rat IL-6 Gene Promoter and Transfection
A genomic DNA clone encoding the rat IL-6 promoter region was obtained by PCR (IL-6 promoter rat sense 5′-GTG GAC AGA AAA CCA GGG AC-3′, IL-6 promoter rat antisense 5′-AGC TGT TCC TGA AGG GCA GAT G-3′), amplifying 1070 bp upstream of the ATG. The PCR product was gel-purified, cloned into pCR II TOPO (Invitrogen, Karlsruhe, Germany) and sequenced. The IL-6 promoter contained various consensus cis DNA elements such as GRE (−559 to −554 bp and −444 to −439 bp), AP-1 (−267 to −260 bp), CRE (−156 to −149 bp), NF-IL6 (−147 to −137 bp), NF-κB (−64 to −53 bp), and the TATA box (−18 to −13 bp). The promoter region of the IL-6 gene was excised from the vector and consecutively digested with SpeI (−758), PvuII (−582), BpmI (−535), DraI (−349 and −254), AatII (−156), DraIII (−149), and MamI (−75). The 5′-ends were blunted by Klenow enzyme or T4 DNA polymerase, and the 3′-ends were digested with XhoI. Deletion mutants were cloned into the SmaI-XhoI site of pGL3 basic vector (Promega, Mannheim, Germany). Site-directed mutagenesis of the AP-1 or CRE binding site was performed using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene Europe, Amsterdam, The Netherlands). All products were confirmed by sequencing. After 48 h in serum-containing medium, IL-6 promoter–firefly luciferase fusion DNA (1 μg) and 0.1 μg of pRL-TK control vector (Renilla luciferase) were introduced to neonatal myocytes with Lipofectamine Plus reagent (Invitrogen). After transfection, the cells were cultured in serum-free CMRL-1415-ATM medium for 24 h, washed twice with PBS, and stimulated with 10−6M isoproterenol, 10−6M nor-epinephrine, or 0.1μg/mL LPS for another 24 h. Luciferase activities were determined using the Dual-Glo Luciferase Assay System (Promega) on a GloMax Luminometer with Dual Injector.
Determination of Transcription Factor Activation in Tissue Lysates
Nuclear protein was isolated from human and mouse LV tissue in a three-step process according to the manufacturer’s instructions (Nuclear Extract Kit, Active Motif Europe, Rixensart, Belgium). Protein concentration was determined by the BCA Protein Assay (Perbio Science Deutschland). Equal quantities of nuclear protein (10 μg) were assayed for CREB, AP-1, and NF-κB binding activity by using the TransAM™ AP-1 c-Jun, TransAM™ CREB and the TransAM™ NFkB p65 Transcription Factor Assay kit (Active Motif Europe) according to the manufacturer’s instructions. All measurements were performed in duplicate and the results are described as percentages relative to the age-matched wild-type mice or nonfailing donor hearts, respectively.
Statistical Analysis
All values are expressed as mean ± SEM. Statistical analysis of differences was done by Student paired or unpaired t-test, by multiple linear regression, or by analysis of variance, followed by Bonferroni-corrected comparisons. Significance was accepted at P < 0.05.
RESULTS
IL-6 Induction in Cultured Cardiomyocytes
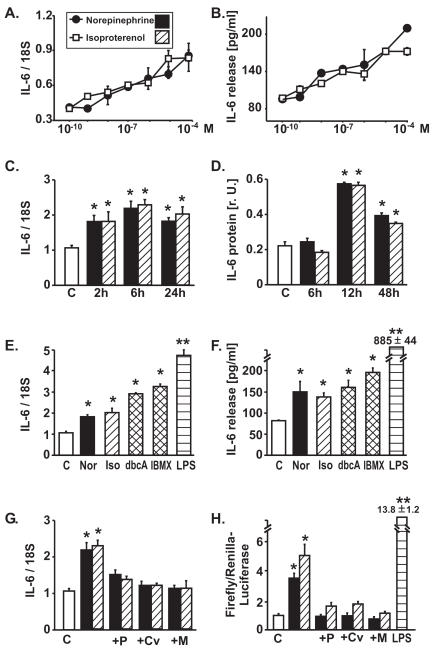
Norepinephrine and isoproterenol induced concentration-dependent IL-6 mRNA expression and IL-6 protein release (Figure 1A and B). This induction was rapid (Figure 1C and D), was mimicked by dbcAMP or by phosphodiesterase inhibition (IBMX) (Figure 1E and F) and was suppressed by β-blockers (Figure 1G and H). Catecholamine-induced IL-6 protein release was moderate (Figure 1B) compared with the release induced by LPS (Figure 1F) or by IL-1β (100 U/mL, 24 h): 2450 ± 213 pg/mL (not shown). Similarly, the catecholamine-induced, β-blocker–sensitive activation of the reporter gene construct (pGL3) containing the IL-6-promoter was moderate compared with the LPS-induced activation (Figure 1H).
Figure 1.
Effect of catecholamines on IL-6 expression in neonatal rat cardiomyocytes. (A) mRNA per Northern blot (densitometric ratios, n = 6 samples per concentration from independent experiments, 12-h exposure), thresholds for significant elevations above control: isoproterenol: 10−9M, norepinephrine: 10−8M. (B) IL-6 protein release (ELISA) into supernatant medium in the same experiments, thresholds as in (A). (C) Time course of IL-6 mRNA induction by norepinephrine (Nor 10−6M) and isoproterenol (Iso 10−6M) (Northern blots, n = 4 per interval). (D) Time course of cellular IL-6 protein content induced by norepinephrine (Nor 10−6M) and isoproterenol (Iso 10−6M) (Western blots, n = 6). (E) Induction of IL-6 mRNA (6 h) and (F) of IL-6 protein release (24 h), induced by norepinephrine (Nor 10−6M), isoproterenol (Iso 10−6M), cell permeable dbcAMP (dbcA 10−4M), IBMX (3-isobutyl-1-methyl-xanthine, 10−4M, phosphodiesterase inhibitor), or LPS (0.1 μg/mL); n = 5 per experiment. (G) Blockade of catecholamine-induced IL-6 mRNA expression (12-h exposure, 1-h preincubation with β-blocker) by propranolol (P, 10−5M), carvedilol (Cv, 10−5M), or metoprolol (M, 10−5M). (H) IL-6 promoter activation in neonatal rat cardiomyocytes transfected with full-length construct, induced by both catecholamines (10−6M, 24 h), is abolished by β-blockers (1-h preincubation with β-blocker, n = 8 per experiment). Note that promoter activation by the inflammatory stimulus LPS (L, 0.1 μg/mL, 24 h) is substantially higher. *P < 0.05 vs. unstimulated control cells, **P < 0.01 vs. unstimulated control cells.
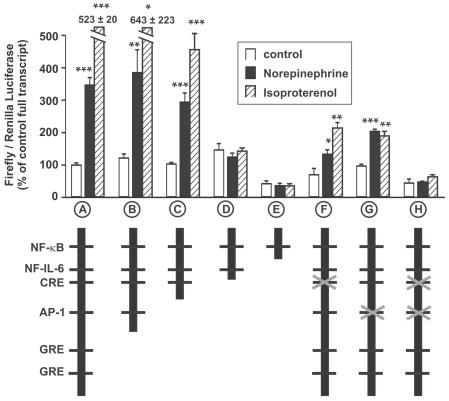
The IL-6 promoter contains various consensus cis DNA elements such as GRE, AP-1, CRE, NF-IL6, or NF-κB. The causal role of CRE- and AP-1- activation for catecholamine-induced IL-6 expression is shown with deletion and mutation analyses of the IL-6 promoter: both AP-1 and CRE binding motifs were necessary and sufficient to obtain this activation (Figure 2).
Figure 2.
Catecholamine-induced luciferase activity in cardiomyocytes transfected with luciferase containing IL-6 promoter constructs. Activation by catecholamines (10−6M, 24 h) in full-length construct-containing myocytes (A) is not attenuated with a construct without glucocorticoid-responsive elements (GRE, B). Induction is still significantly increased when the AP-1-binding motif is deleted (C), but is abolished when both AP-1- and CRE-binding motifs are lacking (D). Further deletion of the NF-IL-6 binding site also attenuates basal activity in unstimulated cells (E). Mutation of binding motifs in full-length promoters by base exchange results in significantly attenuated inductions when the CRE-binding site (F) or the AP-1-site alone (G) are mutated. Induction is abolished when both binding sites are mutated (H). *P < 0.05; **P < 0.01; ***P < 0.001 vs. respective control (unstimulated transfected cells), n = 4–8 independent comparisons per transfection construct.
IL-6 influences cellular and physiological responses via binding to the membrane-bound IL-6 receptor (IL-6R)/glycoprotein 130 (gp130) complex. The soluble IL-6R (sIL-6R) can bind the ligand IL-6 and trigger gp130 signaling in cells that lack the membrane-bound IL-6R, a process named transsignalling. In the failing human heart, multiple components of the IL-6- gp130 receptor system are impaired, indicating that this system has an important role in cardiac pathophysiology. However, we observed no change in the mRNA expression of the membrane-bound and the soluble IL-6 receptor (IL-6R, sIL-6R) or of the signal transducer gp130 in cultured cardiomyocytes under catecholamine stimulation (not shown).
Cardiac Induction of IL-6 by β1-Adrenoceptor Overexpression In Vivo
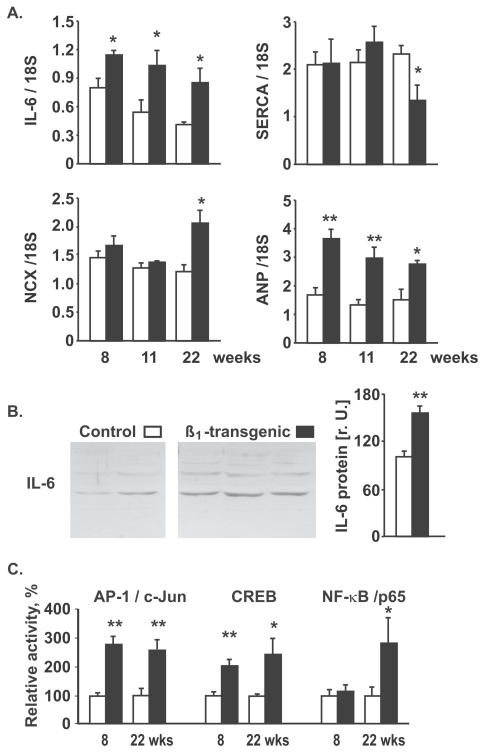
Transgenic mice with cardiomyocyte-specific overexpression of the β1-adrenoceptor demonstrate signs of activated β1-mediated signal transduction and hypercontractility under basal conditions at a young age (<15 weeks) and later develop cardiac failure with marked myocyte hypertrophy (14). We observed increased LV IL-6 mRNA expression in these mice at all ages (8, 11, or 22 weeks) (Figure 3A) and increased IL-6 protein at 11 weeks (Figure 3B). A decrease of SERCA mRNA and an increase in NCX mRNA, resulting in a decreased SERCA/NCX ratio, indicative for transition to heart failure, was detectable only at 22 weeks, but not at younger ages (Figure 3A). At the age of 8 and 22 weeks, transgenic mice exhibited increased CRE- and AP-1-binding activity (Figure 3C), whereas NF-κB activity is was altered only at the age of 22 weeks (n = 5 per group). Thus, chronic activation of β1-mediated signal transduction in cardiomyocytes in vivo results in IL-6 induction via AP-1 and CREB activation, which occurs prior to the transition into heart failure. At all ages, transgenic mice demonstrated a significant increase in proANP mRNA (Figure 3A) but no significant difference in mRNA expression of gp130, IL-6 receptor, phospholamban, and the calcium binding protein S100A1, compared with wild-type mice (not shown).
Figure 3.
. IL-6 induction by cardiomyocyte-specific β1 overexpression. (A) Left ventricular mRNA expression (densitometric relative units) by semiquantitative RT-PCR (normalized to 18S rRNA) of IL-6, SERCA, NCX, and proANP in β1 adrenoceptor overexpressing mice (black columns) and age-matched wild-type mice (open columns), n = 5–10 per group. (B) Left ventricular IL-6 protein expression in 11- week-old β1 transgenic mice and wild-type controls. Note that the two visible bands presumably consist of nonglycosylated IL-6 and glycosylated IL-6 (approximately 21 kDa and 30 kDa); the densitometric sum of both bands was used for quantification. (C) Left ventricular AP-1, CREB, and NF-κB activity as determined by TransAM assays, n = 5 per group; *P < 0.05; **P < 0.01 vs. wild-type.
Induction of IL-6 in End-Stage Human Heart Failure
LV tissue from CHF patients (DCM + ICM) demonstrated increased IL-6 expression (mRNA: 0.24 ± 0.02 vs. 0.17 ± 0.02 in nonfailing donors, P < 0.05; protein: 70 ± 10 rU vs. 36 ± 6 rU, P < 0.05). As a sign of heart failure, mRNA expression is significantly enhanced for proANP and NCX, but reduced for phospholamban and SERCA. No alteration was detected for gp130, IL-6R and sIL-6R (not shown).
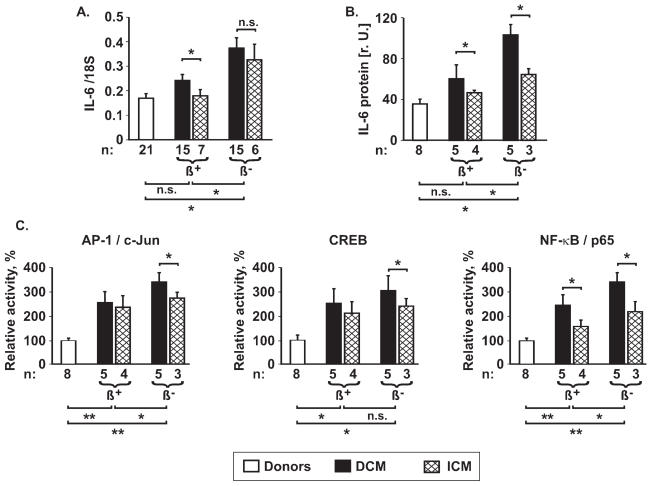
Retrospective subgroup comparisons revealed that ventricular IL-6 expression is higher in patients with DCM than in those with ICM (Figure 4A and B), and that this expression is lowered by therapy with β-blockers (Figure 4A and B). Multivariate analysis by a linear regression model in failing myocardium, considering therapy (β-blockers, ACE-inhibitors) and diagnosis (DCM or ICM), demonstrates an independent effect of β-blockers on IL-6 expression (patients receiving β-blockers vs. patients without β-blockers: P = .002 for mRNA, P = .001 for protein). Similarly, there was a significant difference in IL-6 expression between patients diagnosed with DCM or ICM (P = .09 for mRNA, P = .006 for protein), but there were only minor effects of ACE-inhibition on IL-6 expression (P > 0.10 for mRNA; P = .02 for protein).
Figure 4.
IL-6 induction in human heart failure. Left ventricular IL-6 mRNA (A) was determined by RT-PCR, IL-6 protein (B) was determined by Western Blotting, and AP-1, CREB, and NF-κB activation (C) were examined using TransAM assays in nonfailing donor hearts and in explanted hearts from patients with DCM or with ICM. β+: patients treated with β-blockers prior to explantation; β−: patients treated without β-blockers. Significance of differences: *P < 0.05; **P < 0.01; ***P < 0.001, as indicated. Note that the significant differences shown under the figures describe the comparison between donor hearts, failing hearts from patients receiving β-blocker treatment (DCM + ICM), or failing hearts from patients without β-blocker treatment (DCM + ICM) respectively.
Furthermore, LV tissue from CHF patients demonstrated increased activation of AP-1/c-Jun, CREB, and NF-κB/p65 (Figure 4C), a finding that was even more pronounced in patients without β-blocker therapy compared with patients treated with β-blockers (Figure 4C). Activation of all three transcription factors was higher in nuclear extracts from patients with DCM than in patients with ICM (Figure 4C). All patients had received the β-blockers or ACE-inhibitors for at least one year prior to cardiac transplantation.
DISCUSSION
This study demonstrates β-adrenoceptor mediated myocardial induction of IL-6 via the activation of AP-1-binding sites and CRE under sustained β-adrenergic activation, indicating that activation of AP-1 is an integral component of β-adrenergic signal transduction in cardiomyocytes, parallel to the canonical activation of CRE. The additional activation of NF-κB is demonstrable only in overloaded failing myocardium.
β-Adrenergic Activation of AP-1 and CRE
The promoter analyses (Figure 2) show that mutation of the AP-1- and CRE-binding site results in decreased IL-6 induction, which is further diminished when both sites are abolished. Transcritional stimulation of reporter gene constructs containing the remaining promoter with binding sites for NF-IL-6 and NF-κB by catecholamines shows no difference compared to unstimulated controls, and further deletion of NF-IL-6 also attenuates basal activity in unstimulated cells (Figure 2). Therefore, AP-1 and CRE are necessary and sufficient for norepinephrine- and isoproterenol-mediated induction of IL-6 in cultured cardiomyocytes. In CRE and AP-1 binding site mutation experiments, however, IL-6 induction appeared to be lower (Figure 2H) than when both AP-1-and CRE-binding motifs were lacking in the truncated promoter construct (Figure 2D). We cannot totally exclude an interaction with the remaining consensus cis DNA elements, such as GRE, in the experiments with mutated AP-1 and CRE, although initial experiments did not show a difference between reporter genes including these binding sites and constructs with both GRE elements lacking (not shown).
The concomitant activation of AP-1 and CRE is also the hallmark of myocardial IL-6 induction in the in vivo models of β-adrenergic activation, suggesting that the induction is the direct result of this activation. In young mice with cardiomyocyte-specific overexpression of β1-adrenoceptors (Figure 3), enhanced basal ventricular contractility and heart rate, sensitive to β-blockade, were shown at a young age, whereas cardiac failure develops later in this model (14). At the age of 8 weeks, we observed AP-1 and CRE activation associated with IL-6 expression, whereas NF-κB was activated in the LV at 22 weeks. Parallel with the enhanced IL-6 expression, we observe augmented ventricular proANP expression (Figure 3), consistent with a β-adrenoceptor–mediated contribution to ventricular proANP-induction (15). Similar to the mouse model, failing human myocardium shows an increase in IL-6 mRNA and protein expression as well as increased CRE and AP-1 activity together with additional activation of NF-κB. Thus, activation of CRE and AP-1 is a route of β-adrenoceptor-mediated IL-6 induction in cardiomyocytes in vitro and in vivo. A β-adrenoceptor–mediated IL-6 induction in cardiac fibroblasts, as described by others (6,7), might have contributed to this reaction in cultured cardiomyocytes (due to minimal contamination) and in the myocardium. However, no preferential IL-6 staining of fibroblasts could be demonstrated in cell culture or on tissue slices (not shown).
CRE activation is a known canonical component of cAMP-regulated gene expression, which is still demonstrable in cardiomyocytes with attenuated cAMP-mediated signal transduction due to sustained β-adrenergic stimulation (16). The activation of AP-1 is one final step of the mitogen-activated signal cascade, resulting in DNA-binding of hetero- and homodimers of basic region-leucine zipper proteins from Jun-, Fos-, and other families. A positive interaction of CRE and AP-1 at DNA binding sites has been shown (17), and our data demonstrate that AP-1 activation definitely occurs as part of β-adrenergic signal transduction in cardiomyocytes. Similarly, AP-1 activation via PKA and p38 MAPK is reported to contribute to IL-6 induction in osteoblastic cells under β-adrenergic stimulation (18).
NF-κB Activation and Induction of IL-6
The strong IL-6 induction and NF-κB activation by endotoxin (LPS) in cultured cardiomyocytes (Figure 1) resembles the NF-κB–mediated cytokine expression in immune cells (19). The NF-κB binding site was activated by norepinephrine but not by isoproterenol in isolated neonatal cardiomyocytes (not shown), suggesting β-adrenoceptor–mediated mechanisms. Similarly, Purcell et al. showed that in neonatal cardiomyocytes NF-κB is also activated by various hypertrophic agents including the β1 adrenergic agonist phenylephrine and is required for hypertrophic growth in these cells (20). Furthermore, in vivo experiments also demonstrated the requirement of NF-κB in isoproterenol-induced cardiac hypertrophy (21). In the myocardium in vivo, we saw NF-κB activation only in the LVs of mice with cardiac overexpression of the β1 adrenergic receptor at the age of 22 weeks or in terminally failing explanted human myocardium. However, the transcription factor NF-κB is involved in a variety of physiological and pathophysiological processes, including inflammation, immune response, viral infections, and cell survival responses. In terminally failing myocardium, activation of NF-κB has been observed that is renormalized after hemodynamic unloading by ventricular assist devices (22). This finding argues for a distension-associated activation of NF-κB, either directly or indirectly via distension-mediated induction of TOLL-like receptor-4 as observed in failing myocardium (23). We observed that in failing human myocardium, the IL-6 expression and transcription factor activation is most pronounced in patients without β-blocker treatment prior to explantation (Figure 4), a finding that must not indicate a direct effect of β-adrenergic signaling on NF-κB activity in myocardium. Subtle differences in severity of heart failure among the patients with or without β-blocker therapy cannot be excluded, although the available data prior to heart transplantation did not delineate such differences.
Relevance of β-Adrenoceptor–Mediated Induction of IL-6
IL-6 acts in target cells via hexameric complex formation of two IL-6 molecules each of IL-6-receptor (IL-6R) and gp130. Although the ligand-binding IL-6R alone is not capable of signal transduction, the signaling component gp130 is also involved in the transduction of signals from other ligands of the IL-6 family. When IL-6 is induced by sustained β-adrenoceptor activation, the capacity for signal transduction by IL-6 is probably maintained, because: (a) we found no lowering in the myocardial mRNA expression of IL-6R or gp130 in any model with sustained β-adrenergic activation; (b) in cardiomyocytes, isoproterenol causes a delayed activation of STAT3 (a hallmark of gp130-dependent signal transduction), which can be attenuated by an IL-6-neutralizing antibody (6); and (c) in failing human myocardium, other studies have demonstrated phosphorylation of gp130 and of downstream signaling components (STAT1, STAT3) (24,25).
IL-6 is a pleiotropic cytokine that induces multiple signals in different target cells (26) and has varying effects on myocardial function [recent review in (27)]. In mammalian cardiomyocytes, IL-6 signaling combines inflammatory activation with antiapoptotic survival pathway activation (28), preventing decompensation during cardiac overload (29). On the other hand, IL-6 attenuates β-adrenergic inotropy (30), as do other proinflammatory cytokines (31,32). It has been argued that acutely protective effects of the innate inflammatory stress response convert to maladaptative damage if the inflammatory response is strong and sustained (1,2). Our data show that the β-adrenergic induction of IL-6 in cardiomyocytes is rather low compared with the induction by endotoxin (Figure 1). In in vivo models of chronic β-adrenoceptor activation, however, this induction is sustained and persistent. One crucial determinant for adaptive or maladaptive net effects of IL-6 might be the interaction with other proinflammatory cytokines. Therefore, it will be important to learn whether and which other cytokines besides IL-6 are also induced by the combined activation of AP-1 and CRE, the feature of β-adrenergic signaling in cardiomyocytes.
In summary, we identified AP-1 activation as an integral, contributing part of the signal transduction for β-adrenoceptor–mediated IL-6 induction in cardiomyocytes of nonfailing and failing myocardium, acting in concert with the canonical CRE-activation. In addition, NF-κB is activated in overloaded, failing myocardium.
ACKNOWLEDGMENTS
This study was supported by the DFG (RO 2328/2-1 and SFB 598), by a grant from Byk-Gulden, Ludwigshafen, and by the Wilhelm-Roux-program of the Medical Faculty Halle (FKZ:14/42, FKZ:14/47, FKZ:14/07). The authors are grateful to R Busath and S Koch for technical assistance.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Knuefermann P, Vallejo J, Mann DL. The role of innate immune responses in the heart in health and disease. Trends Cardiovasc Med. 2004;14:1–7. doi: 10.1016/j.tcm.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 3.Sekiguchi K, Li X, Coker M, Flesch M, Barger PM, Sivasubramanian N, Mann DL. Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc Res. 2004;63:433–42. doi: 10.1016/j.cardiores.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Pruett SB. Quantitative aspects of stress-induced immunomodulation. Int Immunopharmacol. 2001;1:507–20. doi: 10.1016/s1567-5769(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 5.Murray DR, Prabhu SD, Chandrasekar B. Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000;101:2338–41. doi: 10.1161/01.cir.101.20.2338. [DOI] [PubMed] [Google Scholar]
- 6.Yin F, Li P, Zheng M, et al. Interleukin-6 Family of Cytokines Mediates Isoproterenol-induced Delayed STAT3 Activation in Mouse Heart. J Biol Chem. 2003;278:21070–5. doi: 10.1074/jbc.M211028200. [DOI] [PubMed] [Google Scholar]
- 7.Yin F, Wang YY, Du JH, Li C, Lu ZZ, Han C, Zhang YY. Noncanonical cAMP pathway and p38 MAPK mediate beta2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol. 2006;40:384–93. doi: 10.1016/j.yjmcc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Briest W, Rassler B, Deten A, et al. Norepinephrine-induced interleukin-6 increase in rat hearts: differential signal transduction in myocytes and non-myocytes. Pflugers Arch. 2003;446:437–46. doi: 10.1007/s00424-003-1043-x. [DOI] [PubMed] [Google Scholar]
- 9.Deten A, Volz HC, Holzl A, Briest W, Zimmer HG. Effect of propranolol on cardiac cytokine expression after myocardial infarction in rats. Mol Cell Biochem. 2003;251:127–37. [PubMed] [Google Scholar]
- 10.Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. beta-adrenergic blockade in developing heart failure: effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation. 2000;101:2103–9. doi: 10.1161/01.cir.101.17.2103. [DOI] [PubMed] [Google Scholar]
- 11.Northemann W, Braciak TA, Hattori M, Lee F, Fey GH. Structure of the rat interleukin 6 gene and its expression in macrophage-derived cells. J Biol Chem. 1989;264:16072–82. [PubMed] [Google Scholar]
- 12.Funakoshi Y, Ichiki T, Ito K, Takeshita A. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension. 1999;34:118–25. doi: 10.1161/01.hyp.34.1.118. [DOI] [PubMed] [Google Scholar]
- 13.Werdan K, Erdmann E. Preparation and culture of embryonic and neonatal heart muscle cells: modification of transport activity. Methods Enzymol. 1989;173:634–62. doi: 10.1016/s0076-6879(89)73042-1. [DOI] [PubMed] [Google Scholar]
- 14.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc Natl Acad Sci. 1999;96:7059–64. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morisco C, Zebrowski DC, Vatner DE, Vatner SF, Sadoshima J. β-Adrenergic cardiac hypertrophy is mediated primarily by the β1-subtype in the rat heart. J Mol Cell Cardiol. 2001;33:561–73. doi: 10.1006/jmcc.2000.1332. [DOI] [PubMed] [Google Scholar]
- 16.Müller FU, Bokník P, Knapp J, Linck B, Lüss H, Neumann J, Schmitz W. Activation and inactivation of cAMP-response element-mediated gene transcription in cardiac myocytes. Cardiovasc Res. 2001;52:95–102. doi: 10.1016/s0008-6363(01)00361-3. [DOI] [PubMed] [Google Scholar]
- 17.Shaulian E, Karin M. AP-1 as a regulator of cell live and death. Nat Cell Biol. 2002;4:E131–6. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 18.Kondo A, Mogi M, Koshihara Y, Togari A. Signal transduction system for interleukin-6 and interleukin-11 synthesis stimulated by epinephrine in human osteoblasts and human osteogenic sarcoma cells. Biochem Pharmacol. 2001;61:319–26. doi: 10.1016/s0006-2952(00)00544-x. [DOI] [PubMed] [Google Scholar]
- 19.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–7. [PubMed] [Google Scholar]
- 20.Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA, Lin A. Activation of NF-kB is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci. 2001;98:6668–73. doi: 10.1073/pnas.111155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freund C, Schmidt-Ullrich R, Baurand A, et al. Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation. 2005;111:2319–25. doi: 10.1161/01.CIR.0000164237.58200.5A. [DOI] [PubMed] [Google Scholar]
- 22.Grabellus F, Levkau B, Sokoll A, et al. Reversible activation of nuclear factor-κB in human end-stage heart failure after left ventricular mechanical support. Cardiovasc Res. 2002;53:124–30. doi: 10.1016/s0008-6363(01)00433-3. [DOI] [PubMed] [Google Scholar]
- 23.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–80. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zolk O, Ng LL, O’Brien RJ, Weyand M, Eschenhagen T. Augmented expression of cardiotrophin-1 in failing human hearts is accompanied by diminished glycoprotein 130 receptor protein abundance. Circulation. 2002;106:1442–6. doi: 10.1161/01.cir.0000033117.39335.df. [DOI] [PubMed] [Google Scholar]
- 25.Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.cir.0000057545.82749.ff. [DOI] [PubMed] [Google Scholar]
- 26.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 27.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: The gp130-STAT3 axis. Basic Res Cardiol. 2007;102:279–97. doi: 10.1007/s00395-007-0658-z. [DOI] [PubMed] [Google Scholar]
- 28.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–83. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 29.Hirota H, Chen J, Betz U, et al. Loss of gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–98. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 30.Müller-Werdan U, Schumann H, Loppnow H, et al. Endotoxin and tumor necrosis factor α exert a similar proinflammatory effect in neonatal rat cardiomyocytes, but have different cardiodepressant profiles. J Mol Cell Cardiol. 1998;30:1027–36. doi: 10.1006/jmcc.1998.0667. [DOI] [PubMed] [Google Scholar]
- 31.Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci. 1989;86:6753–7. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubota T, McTiernan CF, Frye CS, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–35. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]