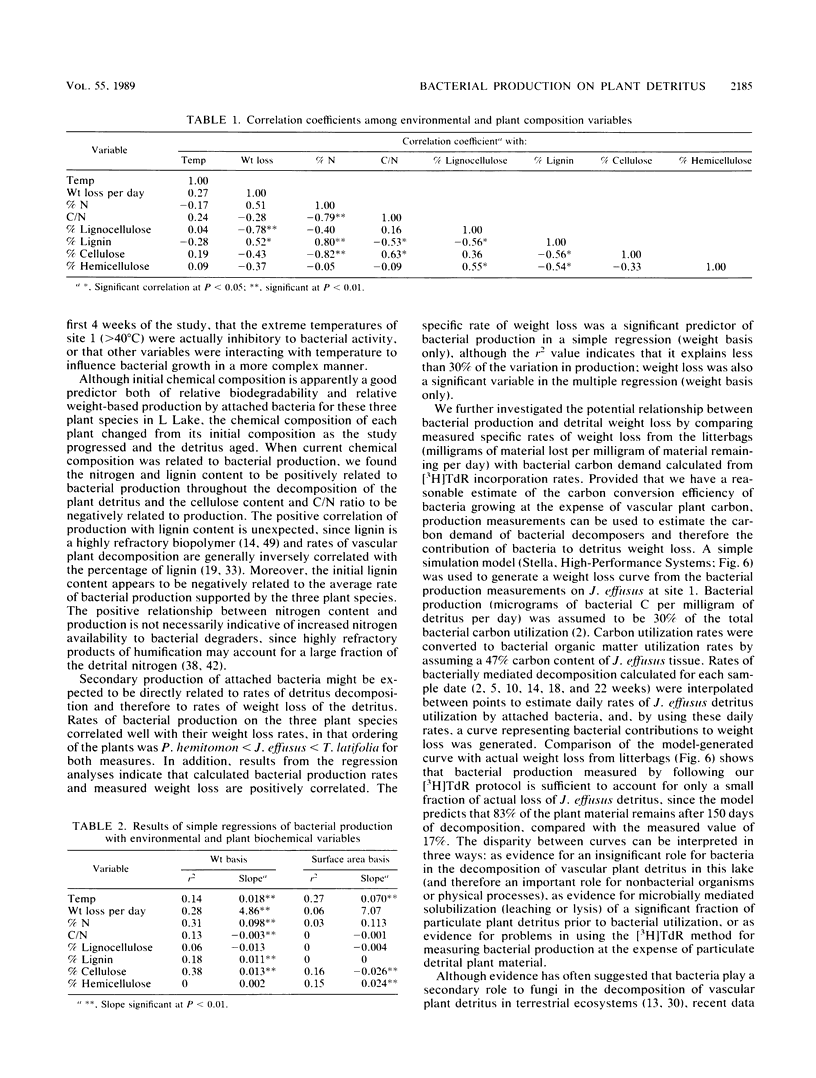
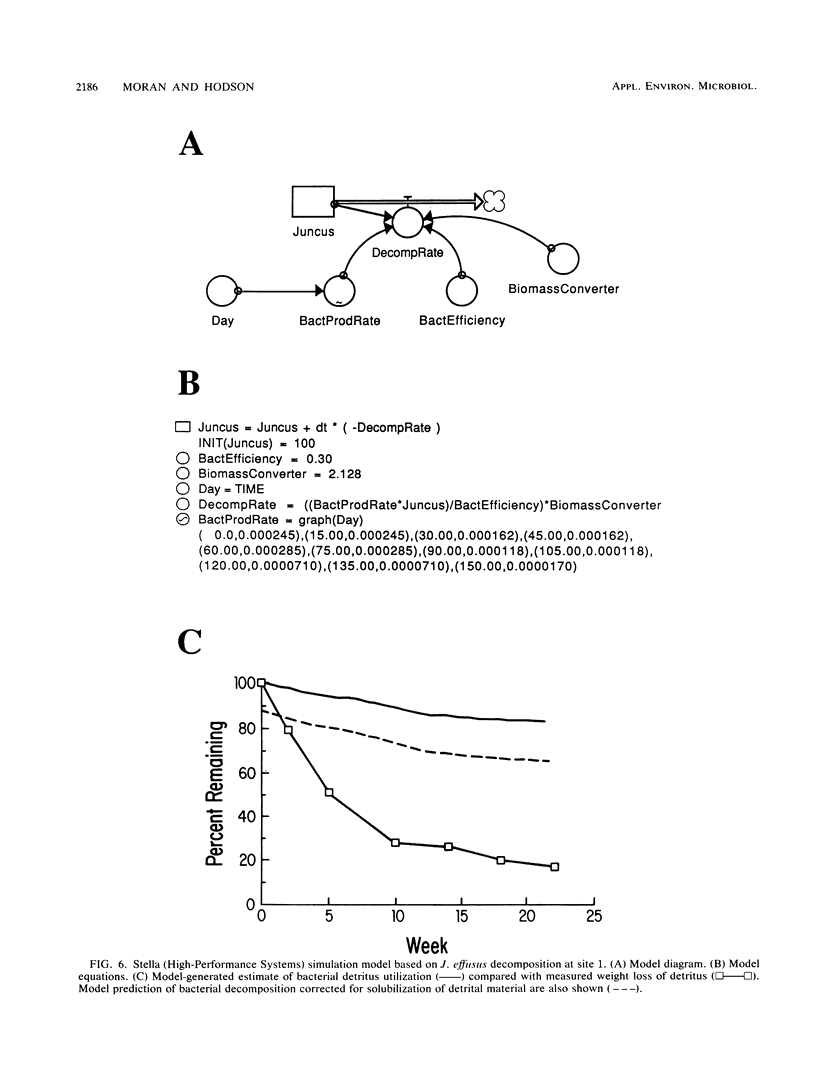
Abstract
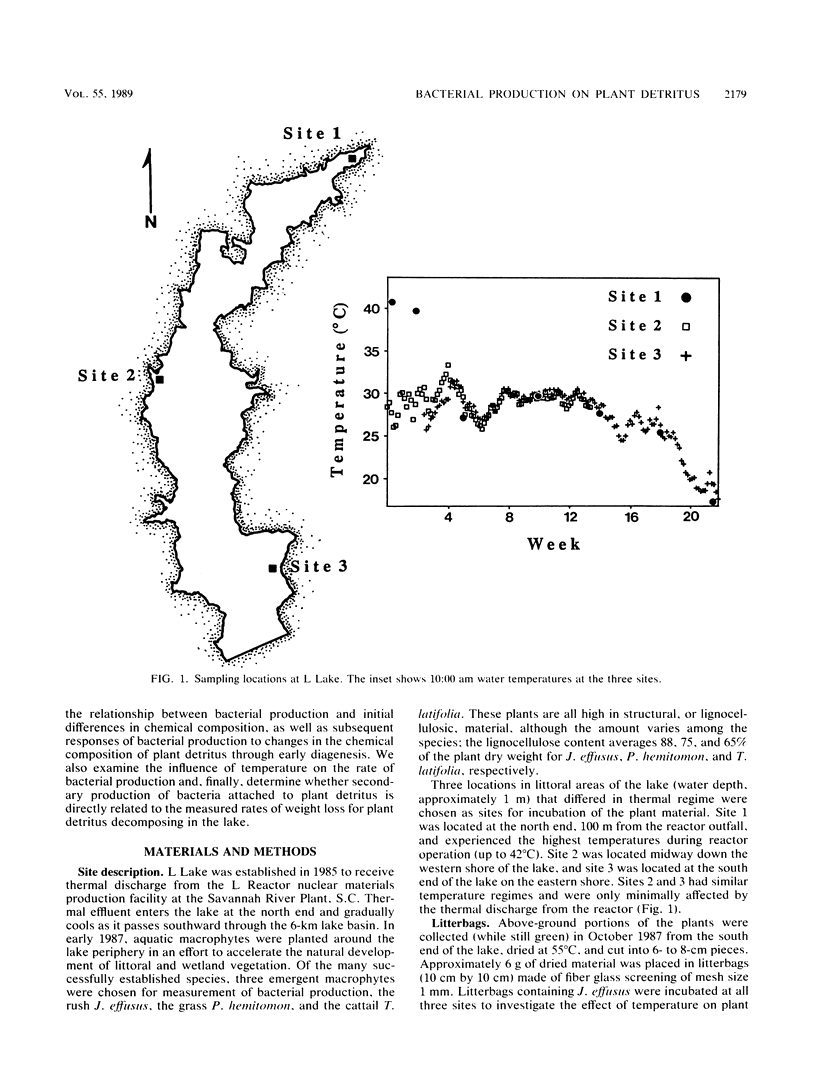
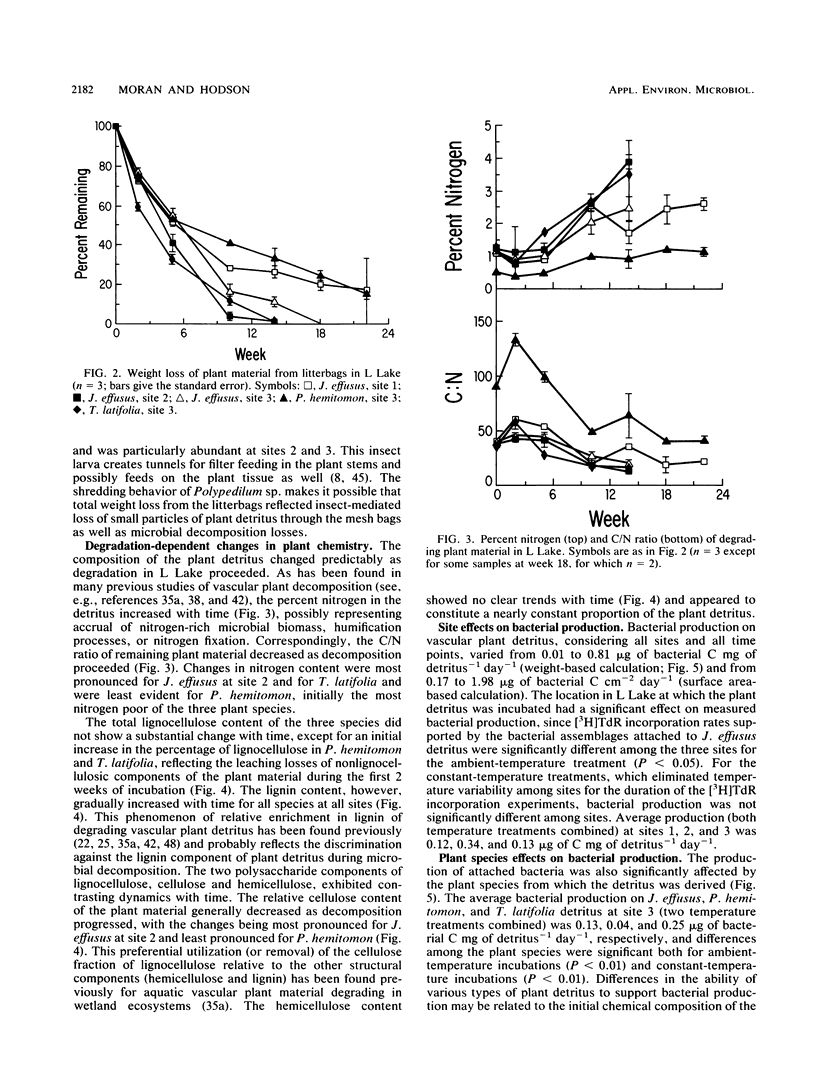
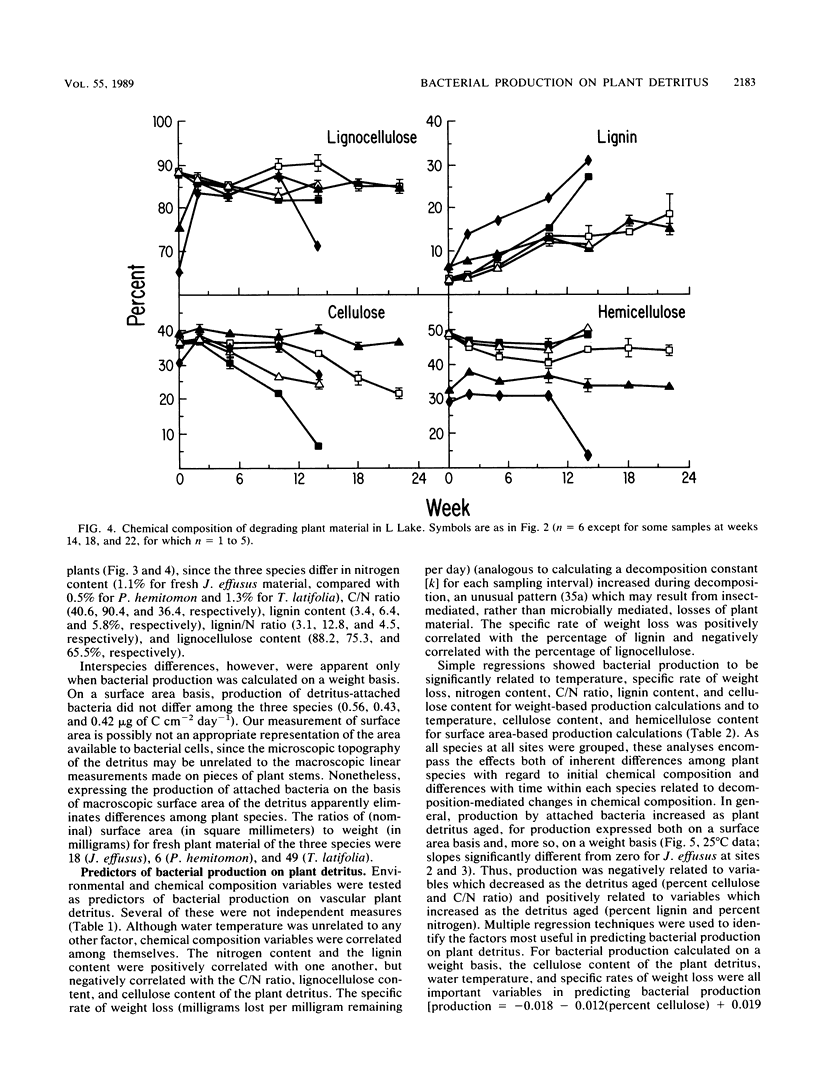
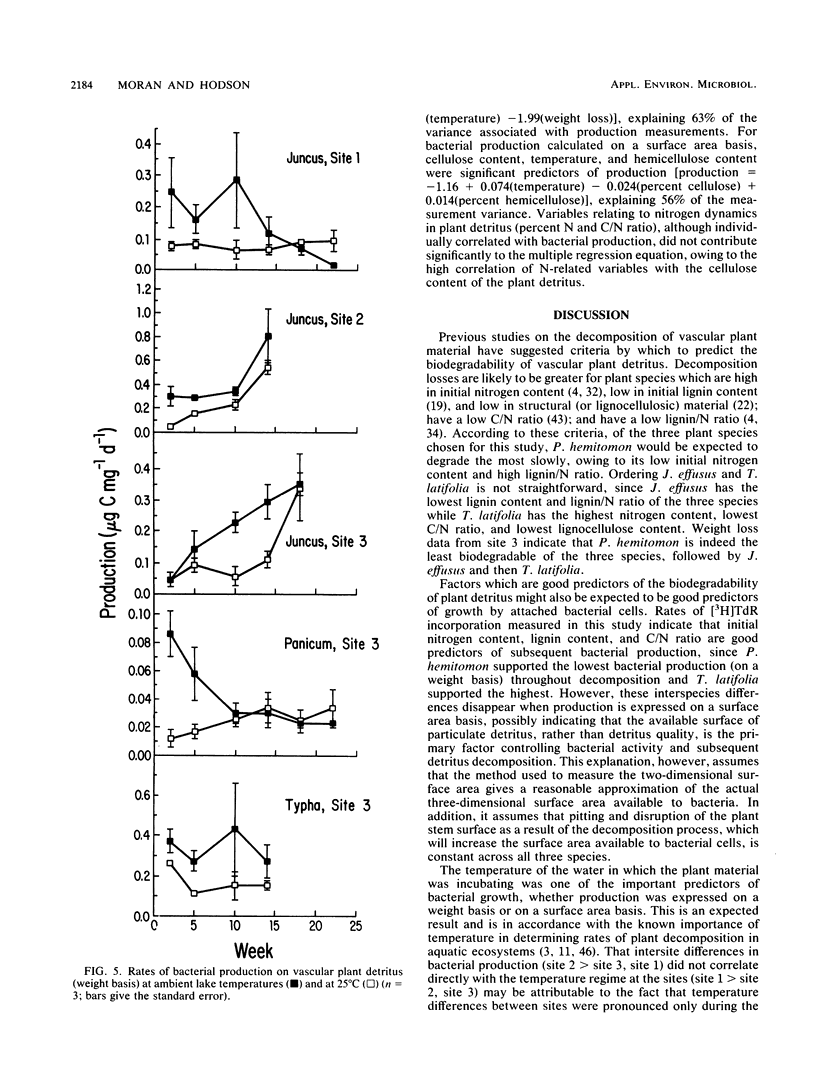
Bacterial production at the expense of vascular plant detritus was measured for three emergent plant species (Juncus effusus, Panicum hemitomon, and Typha latifolia) degrading in the littoral zone of a thermally impacted lake. Bacterial secondary production, measured as tritiated thymidine incorporation into DNA, ranged from 0.01 to 0.81 microgram of bacterial C mg of detritus-1 day-1. The three plant species differed with respect to the amount of bacterial productivity they supported per milligram of detritus, in accordance with the predicted biodegradability of the plant material based on initial nitrogen content, lignin content, and C/N ratio. Bacterial production also varied throughout the 22 weeks of in situ decomposition and was positively related to the nitrogen content and lignin content of the remaining detritus, as well as to the temperature of the lake water. Over time, production was negatively related to the C/N ratio and cellulose content of the degrading plant material. Bacterial production on degrading plant material was also calculated on the basis of plant surface area and ranged from 0.17 to 1.98 micrograms of bacterial C cm-2 day-1. Surface area-based calculations did not correlate well with either initial plant composition or changing composition of the remaining detritus during decomposition. The rate of bacterial detritus degradation, calculated from measured production of surface-attached bacteria, was much lower than the actual rate of weight loss of plant material. This discrepancy may be attributable to the importance of nonbacterial organisms in the degradation and loss of plant material from litterbags or to the microbially mediated solubilization of particulate material prior to bacterial utilization, or both.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benner R., Newell S. Y., Maccubbin A. E., Hodson R. E. Relative contributions of bacteria and fungi to rates of degradation of lignocellulosic detritus in salt-marsh sediments. Appl Environ Microbiol. 1984 Jul;48(1):36–40. doi: 10.1128/aem.48.1.36-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G., Dundas I. Bacterial dry matter content and biomass estimations. Appl Environ Microbiol. 1984 Oct;48(4):755–757. doi: 10.1128/aem.48.4.755-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coveney M. F., Wetzel R. G. Experimental evaluation of conversion factors for the [h]thymidine incorporation assay of bacterial secondary productivity. Appl Environ Microbiol. 1988 Aug;54(8):2018–2026. doi: 10.1128/aem.54.8.2018-2026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. D., Newell S. Y. Thymidine Incorporation by the Microbial Community of Standing Dead Spartina alterniflora. Appl Environ Microbiol. 1986 Nov;52(5):1206–1208. doi: 10.1128/aem.52.5.1206-1208.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. D., Pfaender F. K. Carbon metabolism in model microbial systems from a temperate salt marsh. Appl Environ Microbiol. 1976 Jun;31(6):959–968. doi: 10.1128/aem.31.6.959-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits J. D., Riemann B. Calculation of cell production from [h]thymidine incorporation with freshwater bacteria. Appl Environ Microbiol. 1988 Sep;54(9):2213–2219. doi: 10.1128/aem.54.9.2213-2219.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



