Abstract
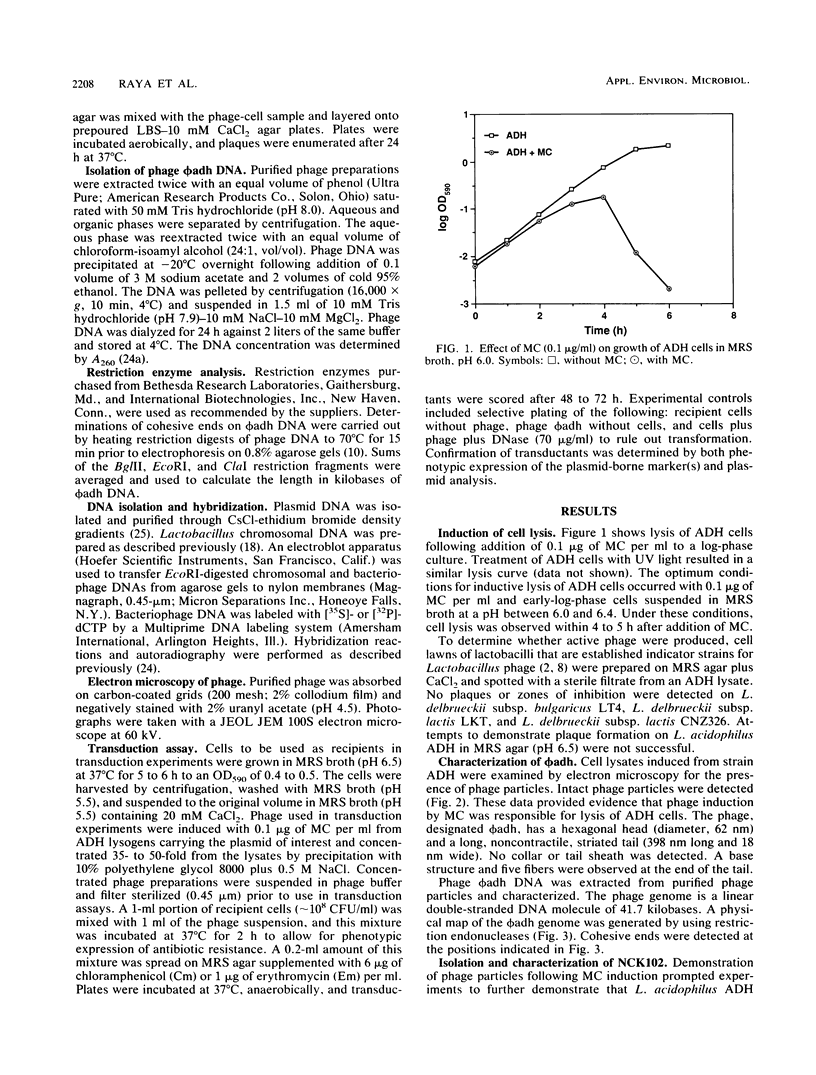
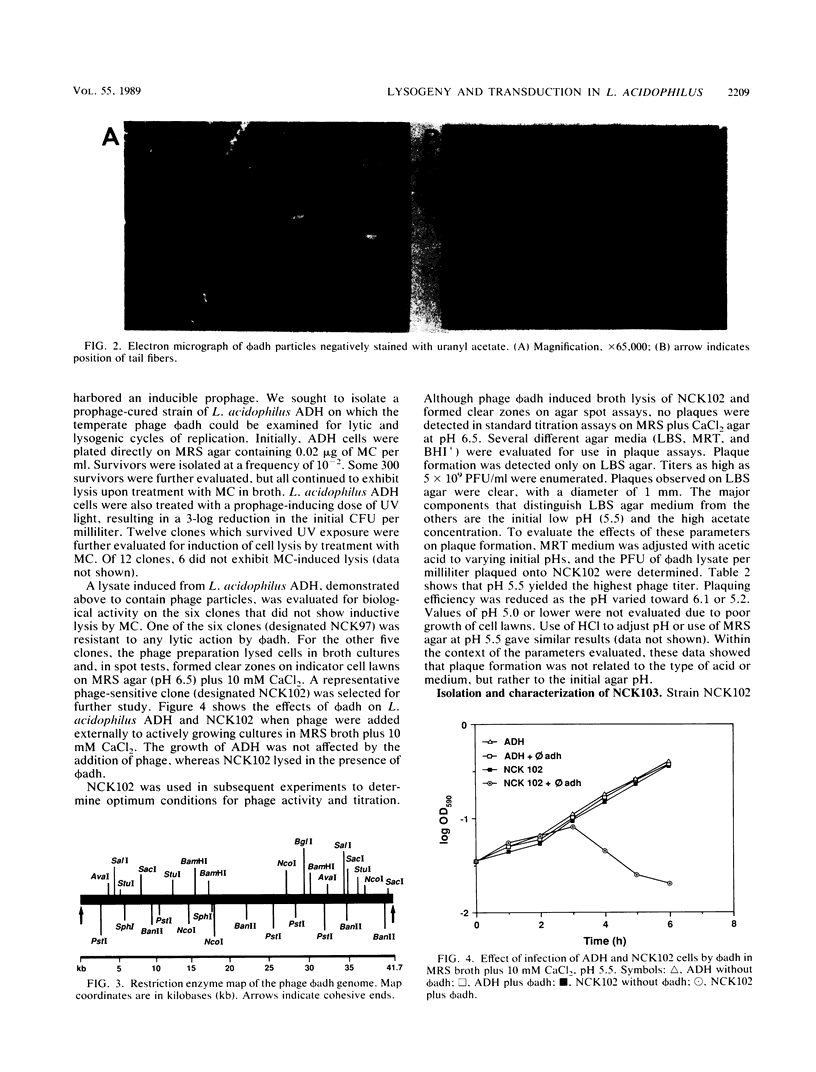
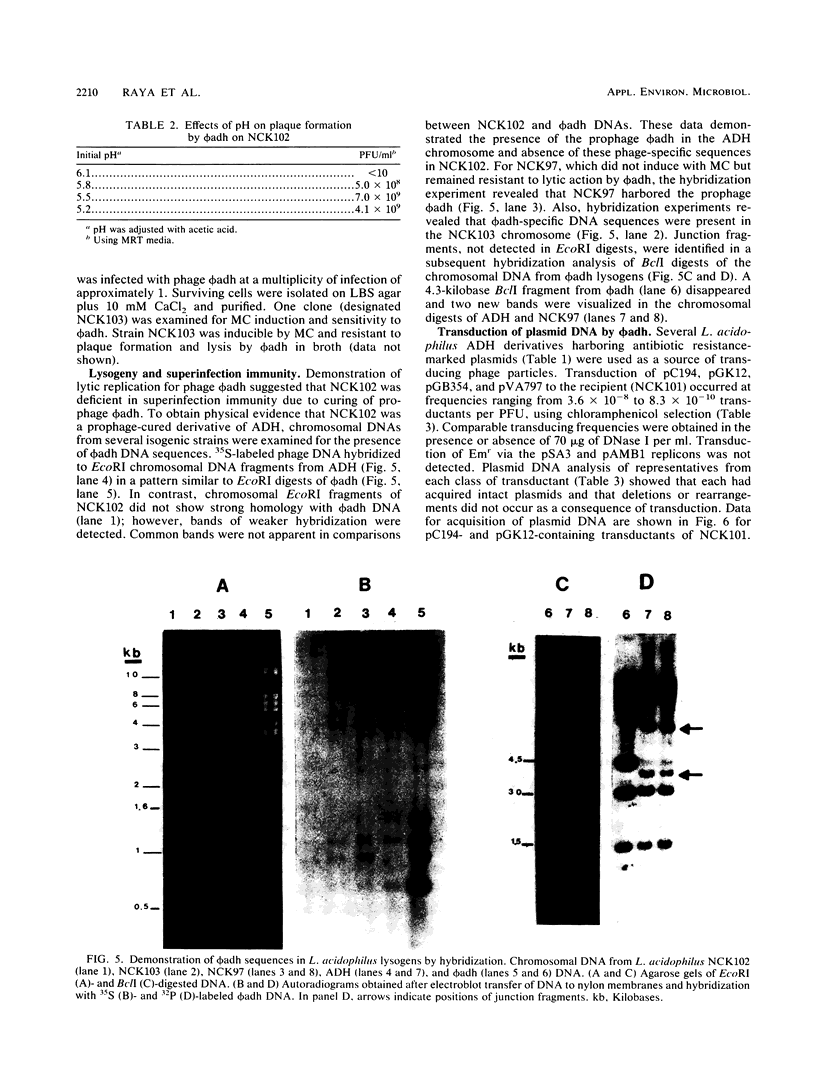
Lactobacillus acidophilus ADH is lysogenic and harbors an inducible prophage, phi adh. Bacteriophage were detected in cell lysates induced by treatment with mitomycin C or UV light. Electron microscopy of lysates revealed phage particles with a hexagonal head (62 nm) and a long, noncontractile, flexible tail (398 nm) ending in at last five short fibers. Phage phi adh was classified within Bradley's B1 phage group and the Siphoviridae family. The phi adh genome is a linear double-stranded DNA molecule of 41.7 kilobase pairs with cohesive ends: a physical map of the phi adh genome was constructed. A prophage-cured derivative of strain ADH, designated NCK102, was isolated from cells that survived UV exposure. NCK102 did not exhibit mitomycin C-induced lysis, but broth cultures lysed upon addition of phage. Phage phi adh produced clear plaques on NCK102 in media containing 10 mM CaCl2 at pH values between 5.2 and 5.5. A relysogenized derivative (NCK103) of NCK102 was isolated that exhibited mitomycin C-induced lysis and superinfection immunity to phage phi adh. Hybridization experiments showed that the phi adh genome was present in the ADH and NCK103 chromosomes, but absent in NCK102. These results demonstrated classic lytic and lysogenic cycles of replication for the temperate phage phi adh induced from L. acidophilus ADH. Phage phi adh also mediates transduction of plasmid DNA. Transductants of strain ADH containing pC194, pGK12, pGB354, and pVA797 were detected at frequencies in the range of 3.6 x 10(-8) to 8.3 x 10(-10) per PFU. Rearrangements or deletions were not detected in these plasmids as a consequence of transduction. This is the first description of plasmid transduction in the genus Lactobacillus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. C., Lüder G., Trautner T. A. Requirements for the formation of plasmid-transducing particles of Bacillus subtilis bacteriophage SPP1. EMBO J. 1986 Dec 20;5(13):3723–3728. doi: 10.1002/j.1460-2075.1986.tb04706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Gilmore M. S. Location of antibiotic resistance determinants, copy control, and replication functions on the double-selective streptococcal cloning vector pGB301. Mol Gen Genet. 1981;184(1):115–120. doi: 10.1007/BF00271206. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COETZEE J. N., DE KLERK H. C. Lysogeny in the genus Lactobacillus. Nature. 1962 May 5;194:505–505. doi: 10.1038/194505a0. [DOI] [PubMed] [Google Scholar]
- Chow J. J., Batt C. A., Sinskey A. J. Characterization of Lactobacillus bulgaricus Bacteriophage ch2. Appl Environ Microbiol. 1988 May;54(5):1138–1142. doi: 10.1128/aem.54.5.1138-1142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17(1-3):1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- Cluzel P. J., Veaux M., Rousseau M., Accolas J. P. Evidence for temperate bacteriophages in two strains of Lactobacillus bulgaricus. J Dairy Res. 1987 Aug;54(3):397–405. doi: 10.1017/s0022029900025577. [DOI] [PubMed] [Google Scholar]
- Coveney J. A., Fitzgerald G. F., Daly C. Detailed characterization and comparison of four lactic streptococcal bacteriophages based on morphology, restriction mapping, DNA homology, and structural protein analysis. Appl Environ Microbiol. 1987 Jul;53(7):1439–1447. doi: 10.1128/aem.53.7.1439-1447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao M. L., Ferretti J. J. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl Environ Microbiol. 1985 Jan;49(1):115–119. doi: 10.1128/aem.49.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downard J. S. Tn5-mediated transposition of plasmid DNA after transduction to Myxococcus xanthus. J Bacteriol. 1988 Oct;170(10):4939–4941. doi: 10.1128/jb.170.10.4939-4941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., Rock M. I., Lee C. Y., Iandolo J. J. Generation of transducing particles in Staphylococcus aureus. J Bacteriol. 1985 Jan;161(1):91–95. doi: 10.1128/jb.161.1.91-95.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. P., Jr, Macrina F. L. Streptococcal R plasmid pIP501: endonuclease site map, resistance determinant location, and construction of novel derivatives. J Bacteriol. 1983 Jun;154(3):1347–1355. doi: 10.1128/jb.154.3.1347-1355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E. M., Chace N. M., London S. B., London J. Transfer of plasmid-mediated antibiotic resistance from streptococci to lactobacilli. J Bacteriol. 1979 Jan;137(1):614–619. doi: 10.1128/jb.137.1.614-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M. C., Klaenhammer T. R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J Bacteriol. 1986 Aug;167(2):439–446. doi: 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L. Isolation and examination of transducing bacteriophage particles from Streptococcus lactis C2. J Dairy Sci. 1976 Mar;59(3):396–404. doi: 10.3168/jds.s0022-0302(76)84219-1. [DOI] [PubMed] [Google Scholar]
- Kleeman E. G., Klaenhammer T. R. Adherence of Lactobacillus species to human fetal intestinal cells. J Dairy Sci. 1982 Nov;65(11):2063–2069. doi: 10.3168/jds.S0022-0302(82)82462-4. [DOI] [PubMed] [Google Scholar]
- Kok J., van der Vossen J. M., Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc D. J., Lee L. N. Physical and genetic analyses of streptococcal plasmid pAM beta 1 and cloning of its replication region. J Bacteriol. 1984 Feb;157(2):445–453. doi: 10.1128/jb.157.2.445-453.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchansky J. B., Benson A. K., Atherly A. G. Construction, transfer and properties of a novel temperature-sensitive integrable plasmid for genomic analysis of Staphylococcus aureus. Mol Microbiol. 1989 Jan;3(1):65–78. doi: 10.1111/j.1365-2958.1989.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Luchansky J. B., Kleeman E. G., Raya R. R., Klaenhammer T. R. Genetic transfer systems for delivery of plasmid deoxyribonucleic acid to Lactobacillus acidophilus ADH: conjugation, electroporation, and transduction. J Dairy Sci. 1989 Jun;72(6):1408–1417. doi: 10.3168/jds.S0022-0302(89)79248-1. [DOI] [PubMed] [Google Scholar]
- Luchansky J. B., Muriana P. M., Klaenhammer T. R. Application of electroporation for transfer of plasmid DNA to Lactobacillus, Lactococcus, Leuconostoc, Listeria, Pediococcus, Bacillus, Staphylococcus, Enterococcus and Propionibacterium. Mol Microbiol. 1988 Sep;2(5):637–646. doi: 10.1111/j.1365-2958.1988.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Mata M., Ritzenthaler P. Present state of lactic acid bacteria phage taxonomy. Biochimie. 1988 Mar;70(3):395–400. doi: 10.1016/0300-9084(88)90213-1. [DOI] [PubMed] [Google Scholar]
- McHenney M. A., Baltz R. H. Transduction of plasmid DNA in Streptomyces spp. and related genera by bacteriophage FP43. J Bacteriol. 1988 May;170(5):2276–2282. doi: 10.1128/jb.170.5.2276-2282.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Efstathiou J. D. Transductional evidence for plasmid linkage of lactose metabolism in streptococcus lactis C2. Appl Environ Microbiol. 1976 Jul;32(1):45–52. doi: 10.1128/aem.32.1.45-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercenier A., Slos P., Faelen M., Lecocq J. P. Plasmid transduction in Streptococcus thermophilus. Mol Gen Genet. 1988 May;212(2):386–389. doi: 10.1007/BF00334713. [DOI] [PubMed] [Google Scholar]
- Morelli L., Cocconcelli P. S., Bottazzi V., Damiani G., Ferretti L., Sgaramella V. Lactobacillus protoplast transformation. Plasmid. 1987 Jan;17(1):73–75. doi: 10.1016/0147-619x(87)90013-8. [DOI] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Conjugal Transfer of Plasmid-Encoded Determinants for Bacteriocin Production and Immunity in Lactobacillus acidophilus 88. Appl Environ Microbiol. 1987 Mar;53(3):553–560. doi: 10.1128/aem.53.3.553-560.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechaud L., Cluzel P. J., Rousseau M., Baumgartner A., Accolas J. P. Bacteriophages of lactobacilli. Biochimie. 1988 Mar;70(3):401–410. doi: 10.1016/0300-9084(88)90214-3. [DOI] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Tsuchida N. Physical mapping of the virion and the prophage DNAs of a temperate Lactobacillus phage phi FSW. J Gen Microbiol. 1984 Feb;130(2):423–430. doi: 10.1099/00221287-130-2-423. [DOI] [PubMed] [Google Scholar]
- Sozzi T., Watanabe K., Stetter K., Smiley M. Bacteriophages of the genus Lactobacillus. Intervirology. 1981;16(3):129–135. doi: 10.1159/000149259. [DOI] [PubMed] [Google Scholar]
- Stetter K. O., Priess H., Delius H. Lactobacillus casei phage PL-1. Molecular properties and first transcription studies in vivo and in vitro. Virology. 1978 Jun 1;87(1):1–12. doi: 10.1016/0042-6822(78)90152-6. [DOI] [PubMed] [Google Scholar]
- Toyama K., Sakurai T., Arai H., Oda A. Studies on temperate phages of Lactobacillus salivarius. I. Morphological, biological, and serological properties of newly isolated temperate phages of Lactobacillus salivarius. Jpn J Microbiol. 1972 Sep;16(5):385–395. doi: 10.1111/j.1348-0421.1972.tb00673.x. [DOI] [PubMed] [Google Scholar]
- Toyama K., Sakurai T., Ari H. [Transduction by temperate phage PLS-1 in Lactobacillus salivarius]. Nihon Saikingaku Zasshi. 1971 Oct;26(10):482–487. doi: 10.3412/jsb.26.482. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yokokura T., Kodaira S., Ishiwa H., Sakurai T. Lysogeny in lactobacilli. J Gen Microbiol. 1974 Oct;84(2):277–284. doi: 10.1099/00221287-84-2-277. [DOI] [PubMed] [Google Scholar]
- de Klerk H. C., Hugo N. Phage-like structures from Lactobacillus acidophilus. J Gen Virol. 1970 Sep;8(3):231–234. doi: 10.1099/0022-1317-8-3-231. [DOI] [PubMed] [Google Scholar]
- van der Vossen J. M., Kok J., Venema G. Construction of cloning, promoter-screening, and terminator-screening shuttle vectors for Bacillus subtilis and Streptococcus lactis. Appl Environ Microbiol. 1985 Aug;50(2):540–542. doi: 10.1128/aem.50.2.540-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]