Abstract
Rapid progress in understanding the molecular mechanisms associated with cochlear and auditory nerve degenerative processes offers hope for the development of gene-transfer and molecular approaches to treat these diseases in patients. For therapies based on these discoveries to become clinically useful, it will be necessary to develop safe and reliable mechanisms for the delivery of drugs into the inner ear, bypassing the blood–labyrinthine barrier. Toward the goal of developing an inner ear perfusion device for human use, a reciprocating microfluidic system that allows perfusion of drugs into the cochlear perilymph through a single inlet hole in scala tympani of the basal turn was developed. The performance of a prototype, extracorporeal reciprocating perfusion system in guinea pigs is described. Analysis of the cochlear distribution of compounds after perfusion took advantage of the place-dependent generation of responses to tones along the length of the cochlea. Perfusion with a control artificial perilymph solution had no effect. Two drugs with well-characterized effects on cochlear physiology, salicylate (5 mM) and DNQX (6,7-Dinitroquinoxaline-2,3-dione; 100 and 300 μM), reversibly altered responses. The magnitude of drug effect decreased with distance from the perfusion pipette for up to 10 mm, and increased with dose and length of application.
Keywords: Hearing, Deafness, Therapy, Pharmacology, Cochlea
1. Introduction
New therapies for treatment of sensorineural hearing loss appear to be on the horizon, based on rapidly accumulating insight into the molecular signals involved in generating new hair cells [1,2], and on progress in understanding the molecular mechanisms associated with cochlear and auditory nerve degenerative processes (e.g., [3-7]; for review, see [7]). Agents have been identified that can minimize degeneration and facilitate repair [4,8-12,14]. Moreover, the extraordinary progress that has been made in defining the genes involved in a number of human genetic forms of deafness [15,16] offers hope for gene-transfer [1,17] and molecular [18] approaches to treat these diseases.
For therapies based on these discoveries to become clinically useful, it will be necessary to develop safe and reliable mechanisms for the delivery of complex compounds into the inner ear. Direct delivery to the fluids of the inner ear is necessary because of the presence of a blood–labyrinth drug barrier, which is anatomically and functionally similar to the blood–brain barrier [19,20]. Direct delivery also has significant potential advantages for therapeutic application. Drugs are largely unaltered by metabolic changes that inevitably occur with other routes of administration, and have ready access to the sensory cells of the inner ear (the hair cells) and the synaptic regions of hair cells (e.g., [21]). Delivery directly to the inner ear can avoid undesirable systemic side effects that some drugs may produce.
Design considerations for an implantable system for human use include a device which will fit within the temporal bone near the cochlea, a high-efficiency, electronically controlled pump used to recirculate inner ear fluid (perilymph), a catheter inserted into the inner ear fluid compartment (scala tympani) through a hole drilled into the bone of the cochlea, a valved drug reservoir, externally programmable controls for delivery of concentrated bioactive compounds, and sensors for detecting and transmitting flow information. The ability to recirculate inner ear fluid through the pump solves several problems. The recirculating fluid permits the reservoir to contain a highly concentrated solution, and thereby potentially operate for years without refilling a reservoir. This eliminates the possibility for microbial contamination during refill. In addition, the perilymph may circulate through the catheter at a rate that is independent of the drug delivery rate. Thus these two flow rates can be optimized separately. It is likely that frequent circulation of the perilymph will maintain patency in the catheter, as opposed to a slow one-way drug perfusion that might operate intermittently. Finally, because there is controlled supply of liquid solvent, the drug storage could take any number of forms such as microchip arrays, bio-erodible polymers, or even hybrid combinations of these drug delivery methods.
The small size and inaccessibility of the cochlea impose constraints on the development of a device to deliver drugs to the inner ear. The cochlea is a coiled, mostly fluid-filled tube with the sensory elements placed on a membrane, which stretches across the middle of the tube. The length of the cochlear duct is around 30 mm in the human and around 18 mm in the guinea pig [22]. Most of the functionally important elements of the cochlea are bathed in perilymph, an extracellular fluid similar to, and in diffusional contact with, the cerebrospinal fluid. Perilymph volume in the guinea pig is 15 μl [23], while that in the human is 160 μl [24]. Toward the goal of developing a chronic inner ear perfusion device for use in humans, a reciprocating microfluidic system that allows perfusion of drugs into the cochlea through a single inlet hole in scala tympani of the basal turn was developed. Infusion and withdrawal through the same hole minimizes the probability of plugging of the perfusion line, since fluid is forced through the tube with each pump stroke at a rate that may be significantly higher than the drug infusion rate.
Assessment of distribution of delivered drug along the length of the cochlea took advantage of the very well-characterized, tonotopic organization of the cochlea, in which responses to high frequency tones arise from the base, low frequency tones from the apex of the coiled tube, with a spatial relationship of about 3 mm per octave [25]. Two indicants of cochlear sensitivity and function were monitored: the outer hair cell-based cochlear distortion product otoacoustic emission (DPOAE), an acoustic signal measured in the external canal in response to bitonal stimulation; and the compound action potential (CAP), an electrical signal evoked through synchronous activation of auditory nerve fibers by tone pips of varying frequency. Two drugs shown previously in constant infusion experiments to influence these responses were chosen for analysis. Salicylate (5 mM) reversibly reduces the DPOAEs, by action on the outer hair cells [26,27]; DNQX (100 and 300 μM) blocks synaptic transmission between the inner hair cell and the afferent nerve fiber to attenuate production of the CAP [28]. Drugs were introduced via an infusion line placed in a single hole drilled in the basal turn. Drugs introduced with the single-input, reciprocating microfluidic system rapidly and reversibly reached cochlear regions significantly apical to the site of entry in scala tympani with no net fluid influx.
2. Methods
2.1. Reciprocating injection system
A reciprocating perfusion apparatus was constructed using commercially available components (with the exception of a simple laboratory-built manifold). The system was designed to inject a small volume (0.5 to a few μl) of liquid into the cochlea over a short (few seconds) time, and to then withdraw the liquid over a period of several minutes. The system was designed to produce no net flow of fluid into the cochlea because preliminary experiments demonstrated that the guinea pig inner ear cannot accommodate significant (more than 5 μl over an hour) addition of fluid without an elevation in thresholds to acoustic stimulation. A schematic representation is shown in Fig. 1. The basic principle of the reciprocating perfusion system is that a pulse is ejected through the discharge capillary and slowly drawn back into the recirculating loop through the following mechanism: compliance and resistance of the return line are adjusted to provide a phase lag between the high pressure pulse at the outlet of the pump and the low pressure pulse at the pump inlet. Consequently with each pump stroke, 500 nl delivered to the manifold is split between the discharge line and the return line. The split is adjustable depending upon the impedance chosen for the return line. The fluid is ejected over a few seconds (Fig. 1) and then returns from the ear to the loop slowly over a period of almost 2 min. The cycle may be repeated at any interval required to achieve the desired fluid delivery rate and circulation rate of perilymph. Multiple pulses may be applied in rapid succession to increase the pulse volume. The result is a system capable of safely accommodating the pulse volumes from the pump, restricting the flow into and out of the ear, and producing no net average flow into the cochlea. Net delivery of dissolved compound is achieved through dilution occurring continuously as the solution is pumped into and back out of the cochlea.
Fig. 1.
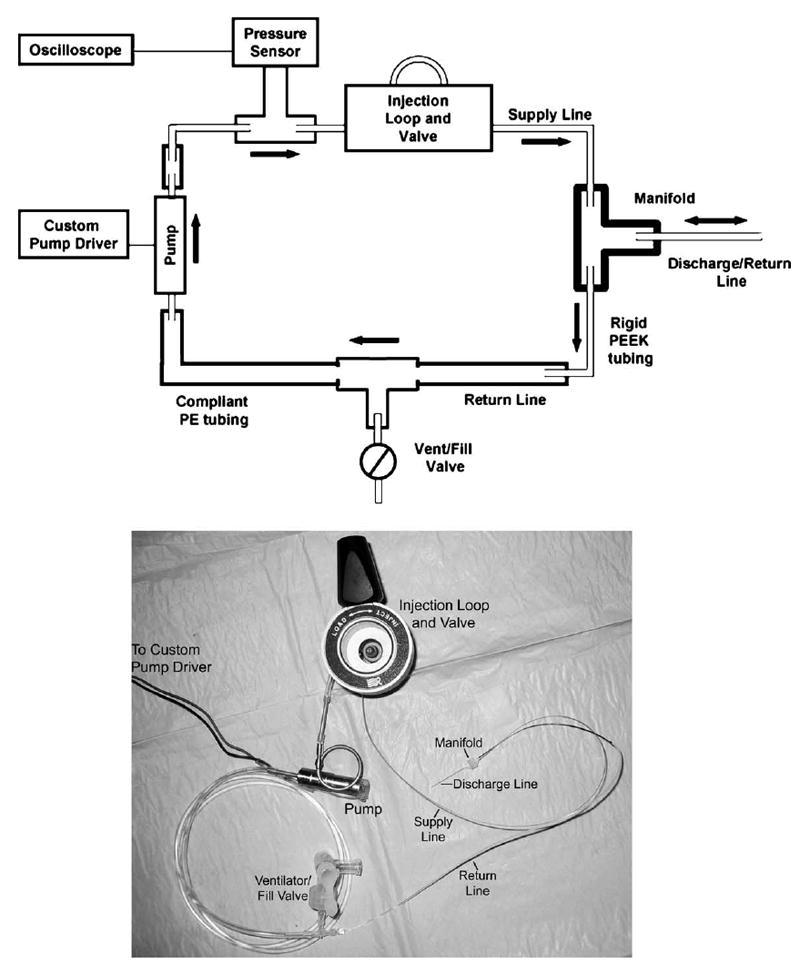
Top panel: System diagram of the recirculating delivery system. Note the intentional use of a long length of compliant PE tubing on the return line. Dimensions are not to scale, but the tubing widths do indicate relative diameters. Bottom panel: Photograph of principal components of the system. Not shown are the electronic controller, the stainless steel sample loop and some components of the injection valve.
The system incorporated a miniature biocompatible reciprocating solenoid pump manufactured by Wilson Greatbatch (Clarence NY) with a stroke volume of 0.5 μl at pressures up to 100 MPa. The pump was controlled with a laboratory-designed electronic controller, which delivered a 15 V pulse to the solenoid at frequencies selectable from 0.1 to 10 Hz. Supply, return, and discharge tubing joined at a 3-port micro-manifold was machined in the laboratory from clear acrylic plastic (see below). Impedance of the fluid lines was controlled by selecting tubing dimensions and materials; a small inside diameter and long length increase resistance, and large diameter, long length, and high elasticity increase compliance. To achieve the desired compliance mismatch, return lines consisted of soft polyethylene tubing, while discharge and supply lines from the pump to the manifold were kept as rigid as possible using either stainless steel or small diameter polyether-ether ketone (PEEK) tubing. By selecting the resistance of the initial part of the return line in relation to the resistance of the discharge capillary, it was possible to set the volume discharged at each outlet cycle. The supply line was PEEK tubing, 28.5 cm long, 152 μm I.D. × 360 μm O.D. The discharge line (to the guinea pig ear) was constructed of piece of polyimide coated fused silica tubing (1.5 cm long, 75 μm I.D. × 144 μm O.D.). The dimensions of this capillary were determined by practical constraints of the surgical procedure. The return line was a length of PEEK capillary tubing (the return “resistor”, 6 cm long, 75 μm I.D. × 360 μm O.D.), followed by a length of compliant polyethylene (PE) tubing (the return “capacitor” 50 cm, 1.27 mm I.D., 2.28 mm O.D.).
To enable the introduction of compounds to the circulating liquid, a high performance liquid chromatography (HPLC) injector (Rheodyne, Rohnert Park CA) with interchangeable, stainless steel sample loops ranging in volume from 20 to 2000 μl was inserted into the supply line. For these acute experiments, the recirculating loop was sufficiently large that sample injected could not cycle through the loop to be reinjected within the time frame of the experiment. A pressure sensor (GE Druck PMP 1210) was fitted at the pump outlet for characterization of the system, but was removed prior to animal procedures to reduce the possibility of introducing air into the system. Pressure measurements were recorded with a data acquisition board.
2.2. Manifold construction
A manifold (3.2 mm by 2.5 mm by 2.3 mm) was fabricated of clear acrylic plastic. Three holes were made with a 0.838 mm drill bit, two on one side and one on the other. The two holes on one side were placed 1.6 mm apart (on center) and the single hole on the other side was drilled such that it intersected with the other two to create a junction between them. The manifold holes were sized such that a short piece of 254 μm I.D. polyethylene (PE10) tubing placed over a piece of PEEK tubing (152 μm I.D., 360 μM O.D.) allowed a tight fit and formed a convenient socket for connecting 75 μm I.D., 144 μm O.D. fused silica tubing. An improved version of the manifold was eventually generated which had similar characteristics to the first manifold, but was less likely to trap air bubbles. This manifold was created with a “U” shaped channel milled into polyimide film using the Quickcircuit precision router system from T-Tech, Inc. Additional polyimide film was layered above and below the milled “U” shape to create a channel. The PEEK tubing was epoxied into the channel. A hole was drilled into the bottom layer of polyimide, intersecting the channel feature, and the fused silica was epoxied into this hole creating a cannula perpendicular to the milled U. The final manifold measured 0.178 × 0.187 in. with a thickness of 0.05 in. This redesign improved the fluidics of the system and facilitated its positioning at the surgical site.
The supply and return tubing were attached to the manifold using Elmer’s Stix-All® and allowed to dry overnight. All tubing, except the discharge tube, and the manifold were filled with de-gassed artificial perilymph (AP) and tested for leaks. De-gassing was accomplished by placing the AP in a vacuum desiccator for several minutes until boiling ceased. A filling/ventilation port was placed on the return side of the recirculating loop. This allowed convenient filling of the loop at the outset of the experiment, cleaning the system at the end of the experiment, and relief of any static pressures within the system before the experiment began.
2.3. Loading and testing perfusion system
Any bubbles in the manifold were removed by “wicking” the liquid from the manifold and then refilling. The discharge tube was then installed in the manifold. Liquid was pumped into the discharge tube before it was glued in place with dental cement (3M ESPE Durelonk™ Carboxylate Cement). Removal of all air from the system was critical for success, both for obtaining repeatable physical performance of the system and because air bubbles injected into the cochlea elevated hearing thresholds. Early prototypes included a pressure sensor at the output of the pump, but this was removed when the system was placed in the animal because of the difficulty in removing all air from the sensor and its associated fittings.
After the dental cement had cured (about 20 min), stability of the configuration was tested. All pressures in the system were allowed to equilibrate by opening a valve that vented to atmosphere. Flow in the discharge tube was calibrated by fitting a 5 cm length of PE10 tubing over the discharge tubing. Fluid was pushed out of the tube until a meniscus could be seen in the PE10 tubing. The pump was turned on for 4 pulses at 2 pulses/s. The distance the meniscus moved before retreating was recorded. The cycle was repeated until the meniscus consistently expanded and retracted to the same point. Typical distances have been 12 mm to 20 mm. With 254 μm I.D. of the PE10 tubing, this converts to volumes of 600 to 1000 μl.
Finally, to simulate delivery of drug into the cochlea with the system, 100 μM fluorescein (Sigma F-6377) dissolved in AP was delivered into a 0.85 mm diameter glass tube while capturing images with a fluorescence microscope (Zeiss Axiovert, FITC filter set) and a 16-bit, cooled CCD camera (Roper, Micromax). This allowed quantification of the reciprocating system’s effective average delivery rate into the reservoir. The fluidic system was filled with AP as described previously. The pump was set to deliver a 600 nl pulse over 3 s, and then to rest for 197 s before repeating. The outlet cannula was fitted into the end of a glass capillary tube (1.5 mm O.D., 0.86 mm I.D.) 10 cm in length. The tube was prefilled with AP and the distal end was open to atmosphere. Calibration of concentration to fluorescence ratios was performed by imaging varying concentrations of fluorescein in identical capillary tubes under identical conditions. Use of 16-bit imaging gave linear relationships for fluorescence over nearly 3 orders of magnitude of concentration. The field of view of the microscope allowed viewing of the first 6 cm of the tube. Images were taken once every second throughout the experiment. The images were analyzed using ImageJ freeware (http://rsb.info.nih.gov/ij/) to obtain the light intensity and hence the concentration of fluorescein along the length of the tube. The software measured the mean intensity of fluorescence in each of six 1-mm segments within the field of view.
2.4. Animals and surgical procedures
All procedures were accomplished in an acoustically and electrically shielded, heated (34 °C) chamber. Seventeen adult albino guinea pigs (Charles River, 350–500 g) were anesthetized with a combination of pentobarbital sodium (Nembutal; 25 mg/kg i.m.), fentanyl (0.2 mg/kg i.m.), and droperidol (10 mg/kg i.m.) with half delivered as an initial bolus and the other half delivered over the next 1 to 1.5 h. Anesthetic boosters (1/4 the original dose) were administered as needed to maintain an adequate depth of anesthesia. The head and neck were shaved, and a small slit was made in the external canal as needed to facilitate clear inspection of the tympanic membrane and placement/coupling of the sound delivery system. The tympani bulla was exposed via a retroauricular incision and an opening on the bulla was created with a sharp blade to visualize the cochlear basal turn and the round window membrane.
Baseline responses (CAP and DPOAE) were monitored as described below. After demonstration of normal baseline sensitivity, a cochleostomy, ~200 μm in diameter, was made adjacent to the round window in the basal turn using a modified pick shaved specifically for this purpose.
After demonstration of proper performance of the pump on the bench with the whole system filled with AP, the fused silica injection tubing was then inserted into the cochleostomy and secured to the bulla with dental cement. After securing the tube to the bulla, fluid was removed near the cochleostomy and the tube was sealed in the cochleostomy with dental cement. The CAP electrode was placed near the round window niche and cemented to the bulla to prevent movement. Hearing was measured after the completion of the surgery, showing slight (generally less than 5 dB) threshold shift to tones of higher frequencies.
Heart rate was monitored throughout the experiment as an indicant of depth of anesthesia. All procedures were conducted with the approval of the Animal Care and Use Committee of the Massachusetts Eye and Ear Infirmary.
2.5. Drugs and drug delivery
Several agents were used as tools to establish the safety and adequacy of the perfusion system. An AP solution served as the control solution and vehicle for the drugs. Its composition was (in mM): NaCl, 120; KCl, 3.5; CaCl2, 1.5; glucose, 5.5; HEPES, 20. The pH was adjusted with NaOH to 7.5 (total Na+ = 130 mM). The experimental drugs, sodium salicylate (5 mM), and the glutamate receptor antagonist DNQX (6-7-dinitroquinoxaline-2,3-dione; 100 μM, 300 μM) were dissolved in the artificial perilymph at desired concentrations and pH-adjusted as needed. DNQX was first dissolved in a small amount of dimethyl sulfoxide (DMSO; final concentration, 0.05%) which does not, on its own, produce significant changes in cochlear responses [28]. Solutions were warmed to near body temperature by placing them in the heated test chamber during surgical preparation and baseline recording activities. All drugs were from Sigma chemicals.
With the perfusion system primed with AP, the pump was turned on with timer control at 4 pulses in 2 s in every 3-min cycle and the round window membrane was visualized through the bulla opening. Bulging of the round window membrane was seen during the pulses, confirming proper functioning of the perfusion system. The AP was delivered for ~50 min and stable hearing was maintained. Then salicylate (5 mM) or DNQX (100 or 300 μM) was loaded into the HPLC injector and injected into the perfusion loop for 85 min, followed by AP washing (80–140 min).
2.6. Stimulus generation and response monitoring
Sound stimuli were created and responses monitored using 16-bit A/D, D/A boards (National Instruments, Austin TX) controlled in a LabVIEW environment by a PC workstation. Signals used to elicit CAPs and DPOAEs were delivered to the ear using the same custom coupler. The coupler accommodates transducers (Tucker Davis EC1) and a Knowles EK3103 electret microphone to measure ear-canal sound pressure (for DPOAEs) via a probe tube concentric with the 2.54 mm (O.D.) sound delivery tube. Sensitivity vs. frequency calibration curves were generated for the monitoring and probe microphones, respectively, enabling conversion from voltage to sound pressure level (SPL; in dB re: 20 μPa). The probe assembly was then placed at the animal’s ear canal where ‘in-animal’ calibration sweeps were accomplished and used to determine the actual SPLs generated at the entrance to the bony canal. After calibration, the probe assembly remained in place for the duration of the experiment.
CAPs of the auditory nerve were elicited using tone pip stimuli (2.78–32.0 kHz; 0.5-ms duration, 0.5-ms rise-fall; cos2 onset envelope; 16/s; 0–80 dB SPL in 5 dB steps). Activity detected locally by a silver wire electrode placed at the round window of the cochlea was amplified (10,000×), filtered (300 Hz to 3 kHz passband), and averaged (32 samples). Response values (e.g., thresholds; peak-to-peak amplitudes) and waveforms were stored to disk. CAP threshold was defined as the lowest stimulus level at which response peaks were clearly and reproducibly present. These visual detection threshold judgments were confirmed following termination of the experiment by offline display and analysis of the stored waveforms.
When two primary tones (f1, f2) are played simultaneously to the ear, acoustic distortion products of their interaction in the cochlea can be propagated back out, through the middle ear, and detected using a sensitive microphone placed in the external ear canal. The distortion product corresponding to the frequency 2f1–f2 is quite robust and is commonly used as a metric of cochlear, and in particular outer hair cell function. These 2f1–f2 DPOAEs present in the canal sound pressure waveform were recorded as response amplitude vs. primary level functions (L1 = 10–75 dB SPL; L2 = L1–10; primaries incremented together in 5 dB steps) spanning the frequency range f2 = 2.78–32.0 kHz (selected to equal CAP test frequencies; f2/f1 = 1.2). Ear-canal sound pressure was amplified, digitally sampled and averaged (25 discrete spectra at each frequency-level combination), and Fast-Fourier transforms were computed from averaged pressures. DPOAE level at 2f1–f2 and surrounding noise floor values (± 50 Hz of 2f1–f2) were extracted. Iso-response contours (L2 levels required to generate a DPOAE amplitude criterion of 0 SPL) were constructed from amplitude vs. sound level data to facilitate comparison with CAPs. This strategy was employed previously to describe protective effects of sound conditioning on CAP vs. DPOAE responses at low vs. high levels of stimulation [29]. Responses were measured at baseline (after bulla opening) and again following cochleostomy and silica tubing insertion and at ~20–40 min intervals during perfusion of pre-drug AP, drug and post-drug AP solutions.
3. Results
3.1. A means of circulating perilymph and delivering drug through a single perfusion line was devised
The performance of the reciprocating fluid system was characterized by simulation and by experiment prior to performing procedures in the guinea pig. Construction of a lumped electrical circuit analog (Fig. 2) allowed simulation of the flow and pressure characteristics using MicroCap software (Spectrum Software, Sunnyvale CA). Fluidic resistance values for the rigid PEEK tubing were calculated using
Fig. 2.
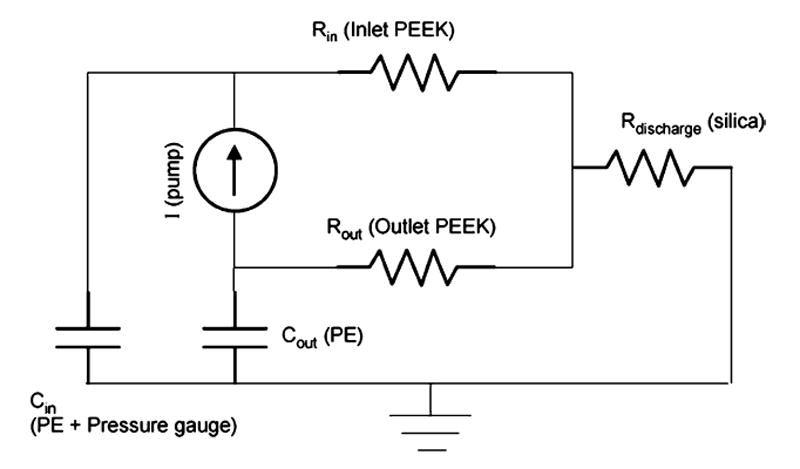
Lumped circuit model simulating the fluid network. Units of resistance are psi min/μl, units of capacitance are μl/psi. The pump is modeled as a current source, the rigid PEEK and silica tubing as resistors, and the compliant PE tubing as capacitors.
where η is the dynamic viscosity of water, L is the tube length and DI is the inner diameter. Capacitance values for the compliant PET tubing were calculated using
where E is the elastic modulus (10 MPa for PE) and t is tubing wall thickness. It should be noted that the pressure sensor itself, because of internal compliance and trapped air, acts as a capacitive element as well. This value of capacitance was experimentally measured (3 μl/psi) and included in the model for simulation.
These results were compared with measurements made by connecting the discharge capillary to an open ended length of tubing and observing the displacement of the water—air meniscus. The motion of the meniscus was captured with a digital imaging system or measured manually against a ruler and converted to a volume displacement using the nominal tubing inside diameter of 254 μm. These results are shown in Figs. 3 and 4.
Fig. 3.
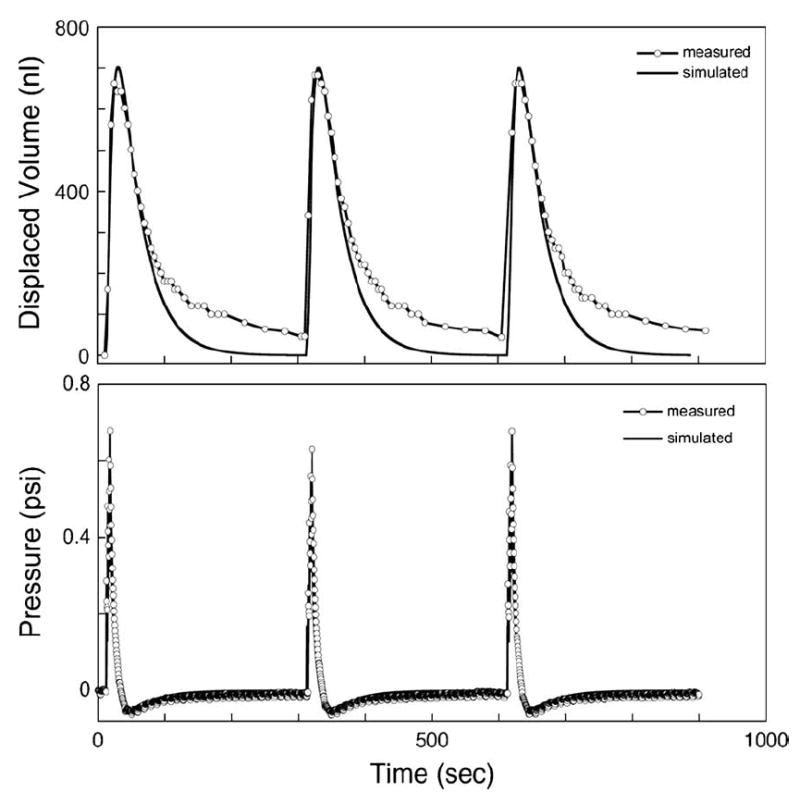
Experimental and simulation data showing the volume output of the reciprocating system when pumping four strokes at 0.5 Hz in 5-min cycles. Note the inlet tube length for this experiment was 3.0 cm rather than 28.5 cm as used for all guinea pig experiments. Of the 2 μl pumped in each cycle, approximately 750 nl is released from the outlet and then withdrawn back into the recirculating loop. The pressure at the outlet of the pump, upstream from the manifold, is also shown. Discrepancies between the data and the simulation probably result because the simulation neglects nonlinear capacitance arising from air bubbles.
Fig. 4.
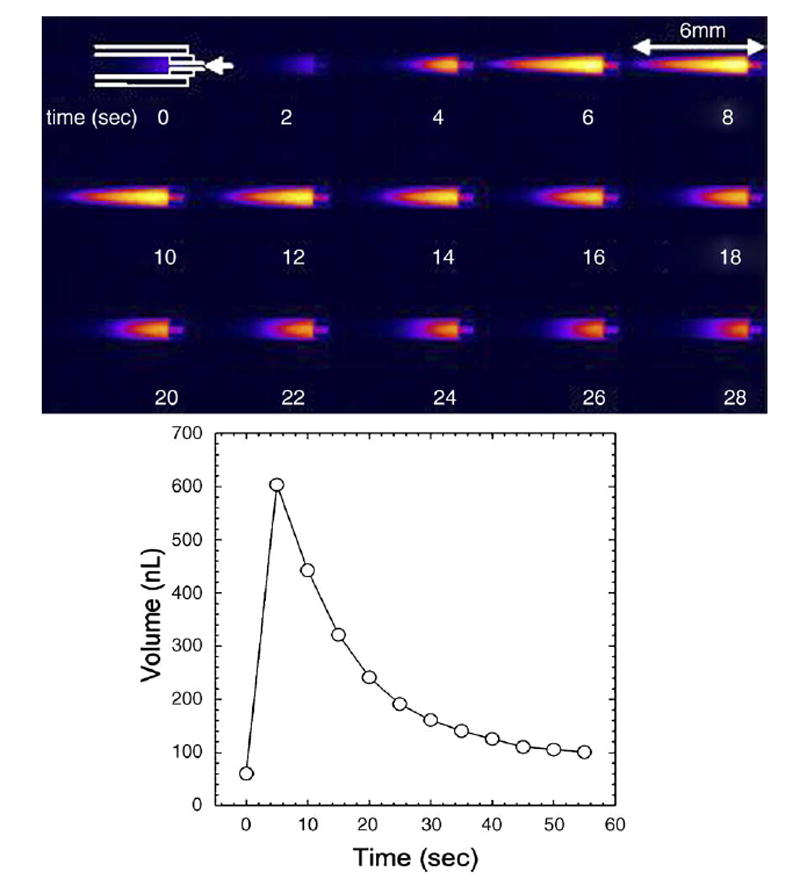
(Bottom) Measured displaced volume over one cycle into empty capillary, and (top) time-step images of output of fluorescein-solution into reservoir tube prefilled with AP. The arrow indicates the location of the outlet of the reciprocating system.
In a typical configuration, the fluid is ejected over a period of several seconds, but then returns from the outlet to the loop slowly over a period of a minute or longer (Fig. 3). The cycle may be repeated at whatever interval is required to achieve the desired circulation rate of perilymph. Selection of the tubing compliance and diameters in the fluid network permitted the pump to operate at a high flow rate (pulsed rates as high as 20 μl/s) while delivering only a fraction of the volume of each pump pulse to the guinea pig ear. Furthermore, the network reduces the peak flow rate, dispersing the initial sharp pulse from the pump into a slow, more benign pulse at the outlet lasting tens of seconds. The volume discharged in each cycle can be increased by delivering multiple pump strokes in rapid succession, at a time scale faster than the response time of the tubing network (e.g., 1 Hz).
Additionally, simulation and measurement verified that the physiological pressures in the cochlea are not sufficient to measurably alter the discharge volume. The discharge capillary was connected to a static pressure head of 20 cm of water and the steady state motion of displacement of a meniscus was monitored and found to be unchanged. Static pressure in the perilymphatic compartment of the cat is around 14 cm of water [30].
3.2. The reciprocating system’s effective average delivery rate into a reservoir was quantified
An experiment was implemented for benchtop measurement of the system during multiple pump cycles. The goal was to measure over time the transport of a compound from the pump loop to an external reservoir and thereby to obtain an effective infusion rate which incorporated both the diffusion and flow mechanisms occurring in the system. Fig. 4 shows a time series of images taken during one infusion cycle to illustrate the fluorescein distribution within the tube. Integrating the concentration at selected time points generated an estimate of the net quantity of fluorescein transferred to the tube over time. The results are plotted in Fig. 5. For the purpose of comparison to constant rate infusion, a linear fit to the first several data points results in an equivalent constant injection rate of 0.012 μl/min of 100 μM solution.
Fig. 5.
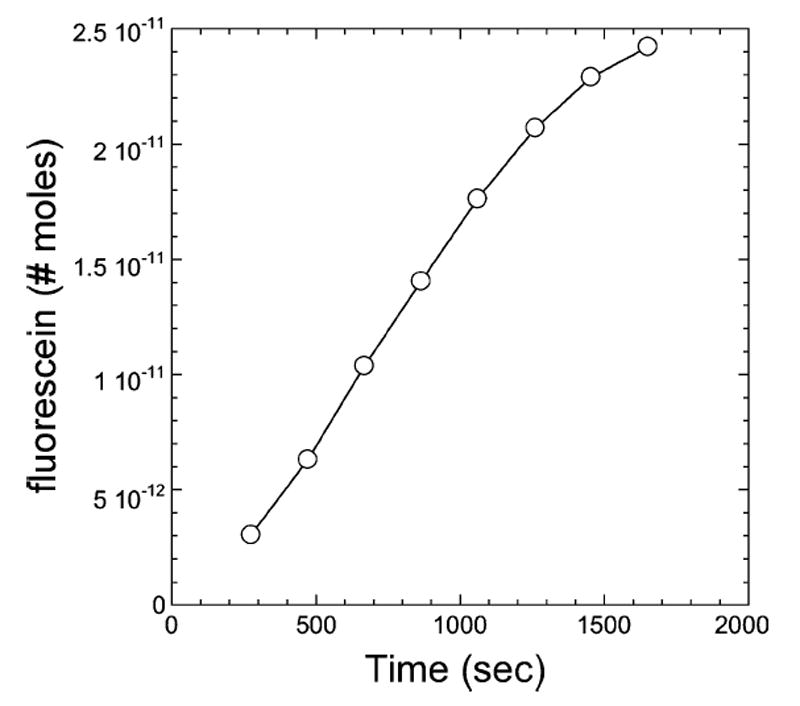
Net quantity of fluorescein transferred into a cylindrical reservoir of AP during the first eight reciprocating cycles.
For drug injection in the guinea pig experiments, flow from the pump to the manifold was diverted into a sample loop to allow injection of drug for a discrete period of time (70 min) followed by a period of wash. Performing a similar procedure with fluorescein and measuring concentration in the capillary tube indicated concentrations of fluorescein during the injection period reached 80% of that in the sample loop in the first 0.5 mm, and 50% at 1.5 to 2 mm, during the period that fluorescein was present at the manifold.
3.3. A cochleostomy can be made, and artificial perilymph perfused, with very little hearing loss
DPOAE and CAP data are plotted in Fig. 6, where responses are shown as a function of frequency of the acoustic stimulus. Drilling the cochleostomy in the guinea pig produced small elevations in threshold at the highest frequencies tested. Threshold elevations were on the order of 5 or 6 dB for the CAP and 3 or 4 dB for the DPOAE. Soon after drilling the cochleostomy, the scala tympani was perfused with AP. In practice, it was necessary to use 3 or 4 pulses over 2 s to drive enough fluid into the cochlea to deliver drugs. A good monitor of fluid flow into the cochlea was to watch movement of the round window membrane during the injection. Slight motion of the membrane (equivalent to that seen during breathing) indicated fluid was flowing into and out of the scala tympani and correlated with good results for drug injection (see below). Threshold measurements after over 33 min of perfusion with an artificial perilymph solution showed little change (Fig. 6). The longest perfusion time was almost 10 h and showed no threshold shifts. Perfusion times in the 17 animals studied averaged 320 min. Average shift in CAP thresholds ranged from less than 2 dB at 2.8 kHz to less than 8 dB at 32 kHz. DP threshold shifts were about half that of the CAP threshold shifts.
Fig. 6.
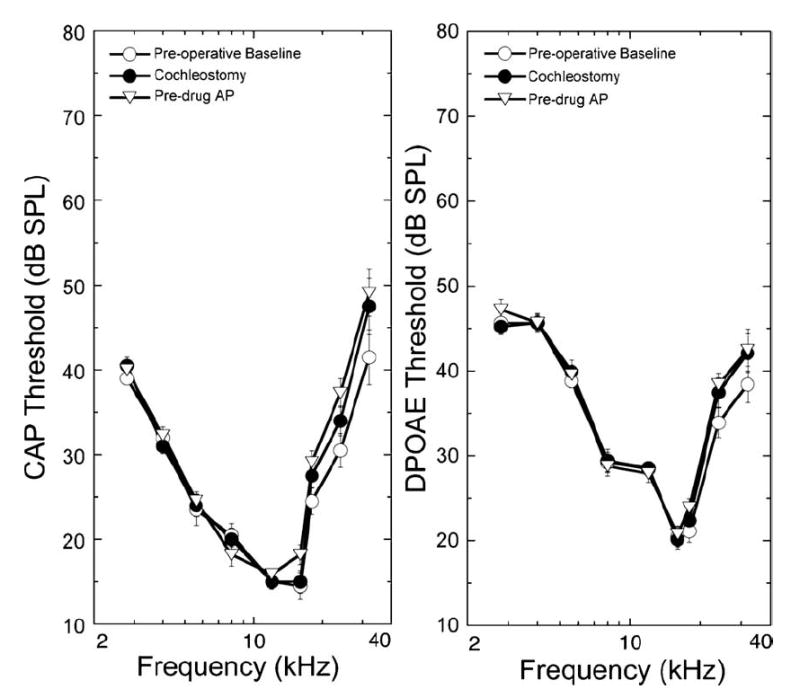
Surgical preparation of the cochlea and perfusion of the control artificial perilymph solution produces minimal changes in low-level CAPs and DPOAEs. Preoperative data were collected after anesthesia, but before any surgery. Post cochleostomy data were taken following drilling of the cochleostomy and placement of the perfusion pipette into the hole. Post AP data were recorded following 33 min perfusions with the control artificial perilymph solution. On this and subsequent figures, DPOAEs are plotted relative to the stimulus level and frequency of the f2 primary required to produce a criterion DPOAE amplitude (iso-response) of 0 dB SPL. The low-frequency differences in the shape of the tuning curves for CAPs vs. DPOAEs are likely related to middle ear filtering of the acoustic DPOAE responses. Data are plotted as means ± S.E. (n = 11 animals).
3.4. DNQX, a glutamate receptor antagonist, affects CAPs but not DPOAEs
To assay the distribution of drugs delivered with the perfusion system, the effects of DNQX, whose action is to block glutamatergic transmission between the inner hair cells and the auditory nerve, were monitored. This action should affect the CAP, which requires synchronous auditory nerve activity, but not the DPOAEs, which are produced by the outer hair cells. Consistent with a site of action at the inner hair cell-afferent fiber synapse, perfusion of DNQX elevated CAP thresholds and reduced suprathreshold amplitudes (data not shown) while leaving DPOAEs unaltered (Fig. 7).
Fig. 7.
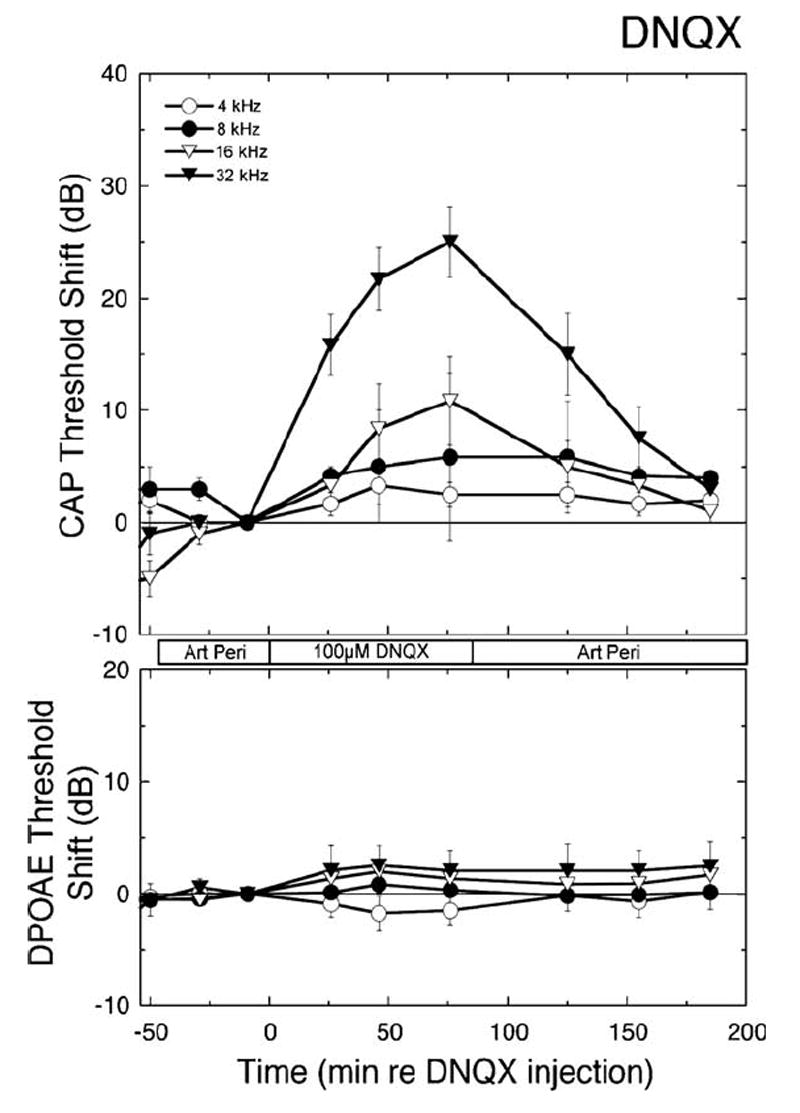
Time dependent changes in CAP thresholds (upper panel) and iso-DPOAEs (lower panel) are plotted as functions of time before, during, and after perfusion of 100 μM DNQX, a glutamate receptor antagonist that blocks transmission between the hair cell and the auditory nerve. Artificial perilymph or DNQX was introduced at the times indicated with the bar between the panels. Times during which data were taken differ slightly because CAPs and DPOAEs were recorded sequentially. Data are plotted as means ± S.E. (n = 5 animals).
Drug effects on CAP were sensitive to stimulus frequency, consistent with the expected distribution of the drug along the length of the cochlea: responses to high frequency stimuli were altered first and most, followed progressively by involvement of responses to mid- and then lower frequency stimuli. The frequency dependence evident in Fig. 7 is explicitly illustrated in the left panels of Fig. 8, where the thresholds during perfusion with 100 μM DNQX are plotted as a function of frequency. Changes were seen as far apically as the 8 kHz region, though they were smaller than those at basal frequencies. Following termination of DNQX perfusion and reintroduction of the AP, responses returned to pre-drug levels. With higher (300 μM) concentrations of DNQX, threshold shifts were observed at frequencies as low as 5.6 kHz (lower panels, Fig. 8). As expected for a drug that selectively blocks synaptic transmission, there were no significant changes in the DPOAEs (right panels, Fig. 8).
Fig. 8.
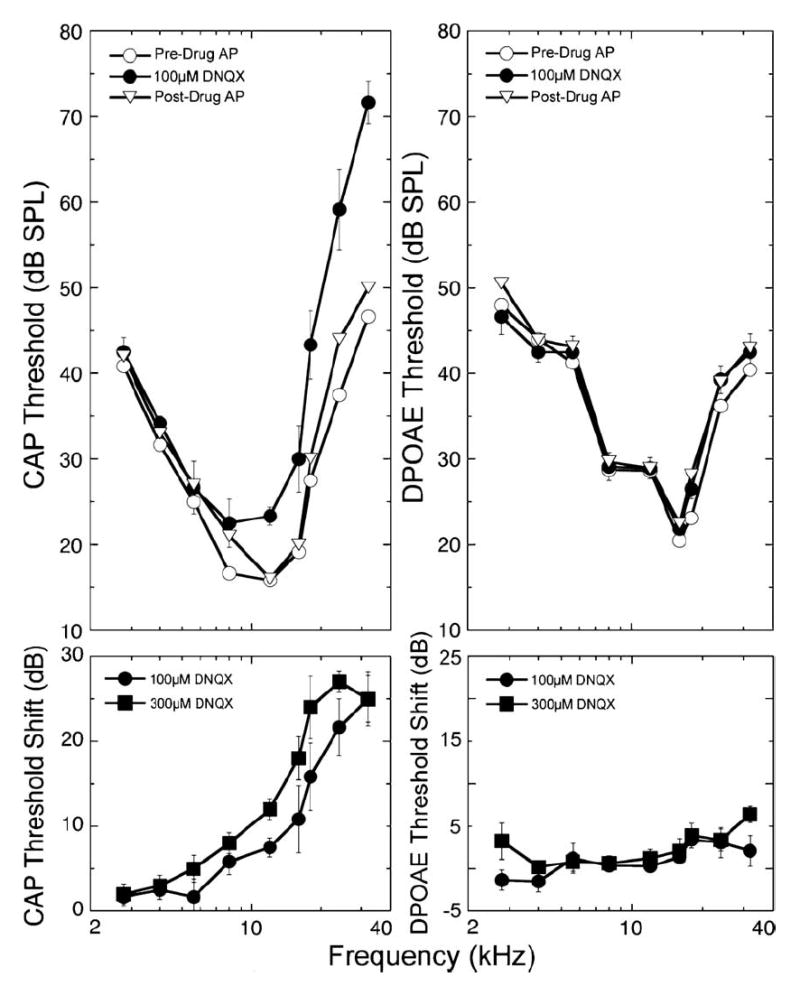
Perfusion of DNQX (100 μM for 85 min) elevated ABR thresholds without affecting iso-DPOAE contours, consistent with its action to block afferent transmission. Effects were greatest at highest frequencies and were reversible with washing. Threshold shifts are indicated in the lower panels, where data for 100 μM DNQX (taken from the panel above) are compared to those with perfusion with 300 μM DNQX. With the higher concentration (300 μM) effects were larger and extended to lower frequency regions of the cochlea (5.7 kHz). Data are plotted as means ± S.E. (n = 5 animals).
3.5. Salicylate perfusion reversibly affected both CAP and DPOAEs
Salicylates are well known to interfere with outer hair cell electromotility [31] and would be expected, therefore, to alter both DPOAEs and CAPs (with CAP alteration secondary to an effect on the hair cell). Consistent with this expectation, salicylate altered both CAP thresholds and low-level iso-DPOAEs, as displayed in Fig. 9. With perfusion of 5 mM salicylate for 85 min, effects were observed as far apically as 5.6 kHz, though effects were largest at higher frequencies. The time course of the effect of salicylate perfusion was similar to that seen with DNQX. Highest frequency regions were affected first, followed by threshold elevations for more apical regions later. Note also that the responses at higher frequencies began to reverse more quickly, consistent with the idea that the content of the scala tympani near the basal turn is being washed out with the artificial perilymph solution. Fig. 10 illustrates CAP threshold and iso-DPOAEs as a function of frequency before, during, and after perfusion with 5 mM salicylate. These effects of intracochlear salicylate perfusion are similar to those observed previously during acute perilymphatic perfusion of salicylate in the guinea pig with a traditional two hole perfusion system [27]. The peak effect was seen at 24 kHz, though this is probably more a reflection of the stimulus level ceiling in the acoustic system, which was reached for the 32 kHz responses.
Fig. 9.
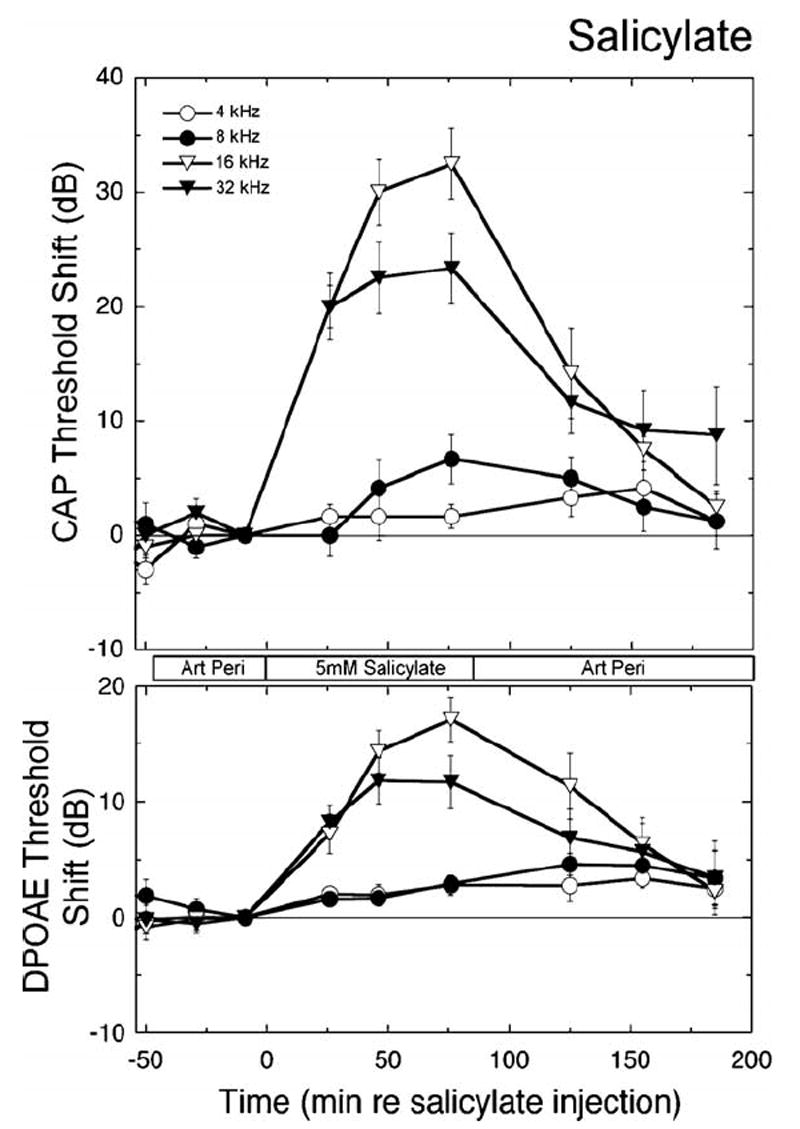
Perfusion of salicylate. Salicylate (5 mM) reversibly elevated CAP thresholds and low-level iso-DPOAE contours. Highest frequencies were affected most and earliest, consistent with the basal site of drug entry. The magnitude of the high frequency shifts in DPOAEs are underestimated in this plot, because post-drug responses in many animals reached the stimulus level ceiling for frequencies above 24 kHz. Data are plotted as means ± S.E. (n = 6 animals).
Fig. 10.
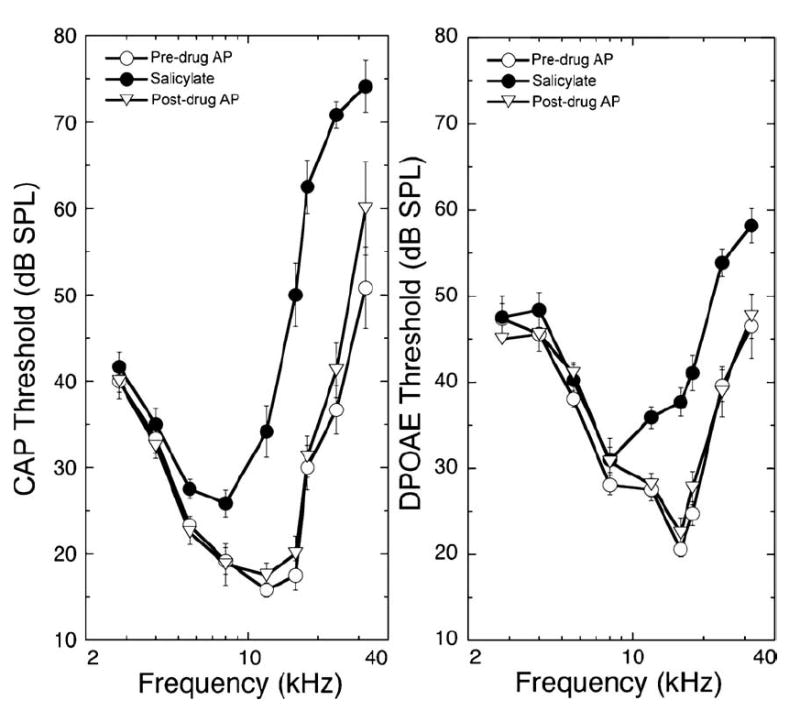
CAP thresholds and DPOAE iso-response values (re: 0 dB SPL) are shown before and after perfusion of control and 5 mM salicylate as functions of frequency. Data plotted are mean ± S.E. for 6 animals.
4. Discussion
The analysis demonstrated that it is possible to deliver drugs into the cochlea via a single injection capillary using a reciprocating perfusion system designed to operate via a rapid fluid pulse into the cochlea and a slow return. Even with the relatively short perfusion times (85 min) used in this study, it was possible to deliver agents as far apically as the 6 to 8 kHz region. It is very likely that with longer perfusion times and, as demonstrated, with higher drug concentrations, drugs will be delivered even more apically. The method of perfusion is relatively innocuous and stable, producing little hearing loss over the 4 to 10 h time periods we perfused the cochlea.
An early design decision was to recirculate perilymph through the pump and to deliver drugs into the recirculating fluid. One consideration was a doublelumen microcatheter with a separate inlet and outlet. However, a reciprocating (perfuse/withdraw) scheme through a single line seemed more likely to reduce the probability of plugging of the perfusion line, since fluid is forced through the tube with each pump stroke at a flow rate that may be significantly higher than perfusion rate with more traditional techniques. Recirculation of drug after injection was not an issue in these acute experiments because the recirculation loop was sufficiently large to prevent recycling over the time course of these acute experiments. In chronic preparations, drug injected will likely be recycled multiple times before it is cleared, by the inner ear, from the fluid network.
4.1. Distribution of drug along cochlea
In essence, monitoring cochlear responses to low level tones served as a bioassay for drug distribution of salicylate and the glutamate receptor antagonist, DNQX. Because cochlear responses reflect activity in a discrete region of the cochlea, and the frequency-to-place map of the guinea pig cochlea is well characterized (reviewed and summarized in [25]), fairly accurate determination of the distribution of the drug along the length of the cochlea was possible. As expected, the specific glutamatergic antagonist, DNQX, altered the CAP, but not the outer-hair-cell-based DPOAEs. Salicylate, on the other hand, altered the outer hair cells and thus affected both the DPOAE and the CAP. DNQX is an especially good monitor for these characterizations, serving as an internal control for possible nonspecific effects of the perfusion since it does not alter the DPOAE.
The fluorescein experiments indicate that concentrations building up in a capillary tube require relatively long times to equilibrate with the concentration of that in the sample loop. This is not necessarily a drawback, as gradual administration of drugs is often desirable. It is also notable that, in comparison with simulations of constant infusion of an equivalent quantity of fluorescein, the results suggest that the rate of transport of compound toward the distal end of the tube is enhanced by the reciprocating flow.
It is recognized that a cylindrical rigid capillary is a crude approximation of the physiological complexity of the cochlea. A glass capillary of 0.85 μm diameter is of similar cross sectional area of that of the basal 4 mm of the scala tympani of the guinea pig cochlea (see Salt et al. [22]) though the cochlea narrows considerably beyond 4 mm. It will be possible to continue these studies in reservoirs with more sophisticated geometry and dynamics.
4.2. Comparison to other delivery methods
The practice of placing drugs of interest within cochlear perilymphatic spaces via a perfusion technique is a method with a long history of successful application (e.g., [32-42]). When carefully administered, the technique itself has been shown to have little effect on a variety of gross cochlear and neural potentials as recorded from sites within and near the cochlea (e.g., [43]). This mode of delivery bypasses the blood–cochlea barrier, allowing drugs to reach their intended targets more directly with lower doses and fewer nonspecific actions. Drugs are largely unaltered by metabolic changes that inevitably occur with other routes of administration. Drugs perfused into the perilymph compartment of scala tympani have ready access to the hair cells and the synaptic regions of hair cells, a view supported by investigations in which various stains demonstrated ready access to structures within the organ of Corti when introduced via the scala tympani perilymph compartment (e.g., [21]). Additionally, a comparison of the concentrations of cholinergic antagonists required to block the cochlear efferents in vivo [41,42] to those effective at in vitro isolated outer hair cells [44] shows remarkably close agreement.
Most methods of perfusion of the guinea pig scala tympani are derivations of Konishi’s technique, in which an inlet hole is placed in the scala tympani and an exit hole is placed either in the apex of the cochlea or in the scala vestibuli (e.g., [32-42]). This allows reasonable control of drug concentration and agents can be distributed throughout the length of the cochlea. A hole in the apex usually compromises cochlear function in the very apical regions of the cochlea, but neural responses from that region are usually not monitored because of difficulty in getting a synchronous extracellularly monitored response from low characteristic frequency neurons. Based on experience with both approaches, it would appear that for acute delivery of drugs to the inner ear, the use of a reciprocating perfusion mechanism may offer slightly better stability of cochlear responses, but sacrifices ability to deliver drugs to more apical regions of the cochlea over the relatively brief time course of an acute experiment.
Another approach is to use an osmotic pump to deliver drugs and fluid into a cochleostomy. Investigators at Kresge Hearing Research Institute, University of Michigan have developed and tested extensively a method of osmotic pump-driven drug delivery via a cochleostomy into the fluid space of scala tympani of the cochlear basal turn (e.g., [45-48]). Such a pump can provide more or less continuous delivery for periods of up to several weeks. Their work has shown that flow rates of ~0.5 μl/h preserve the delicate structures and functions of the cochlea while delivering sufficient drug to produce intended effects. Indeed, it has been suggested that higher rates of infusion are not only ineffective in improving drug distribution within the cochlear partition; they result in displacement of the infusion solution through the cochlear aqueduct and into the CSF [49]. In the hands of the Michigan group (e.g., [47]), ABR thresholds from 2 to 20 kHz remained within ~5 dB of pre-perfusion levels after 3 days of continuous delivery. Infusions reportedly have been successfully accomplished for as long as 60 days, although no details on cochlear function or histology are provided. Carvalho and Lalwani [50] also have undertaken continuous osmotic pump mediated intracochlear perfusion in guinea pig and report good preservation of hearing in low and mid frequencies and mild threshold elevations at frequencies >16 kHz at 3 and 7 days after the start of perfusion. Potential problems with osmotic pumps for possible clinical application include difficulty in precisely controlling flow rates and the inability to noninvasively start and stop infusion.
Currently, otologic practice relies heavily on both systemic and middle ear drug delivery routes. Drugs are commonly delivered systemically, with the hope that they will find their way to their intended inner ear targets in the form and concentration desired, and without serious side effects. Systemic corticosteroids, for example, are used in the otologic management of idiopathic sudden and immune-mediated sensorineural hearing losses (e.g., [51-53]). Their clinical usefulness, however, is limited by undesirable side effects arising from the high systemic doses required to achieve sufficient cochlear fluid levels of drug to produce the intended inner ear effects [54]. Middle ear application has advantages over systemic drug delivery, in that drugs so applied can reach their desired targets at higher concentrations and with fewer unwanted systemic side effects. The application is straightforward, and complications are minimal. A major limitation of these methods, however, is the inability to precisely control the amount of drug that diffuses from the middle ear through the round window membrane into the inner ear. Individual variation in mucous membrane thickness, mucosal folds and middle ear anatomy can have a significant impact on the amount of drug that ultimately enters the inner ear. Plontke and Zenner [55] report, for example, round window niche obstruction in 33% of human ears. This becomes even more problematic when considering delivery of complex macromolecules with limited diffusion coefficients [56] and those requiring sequenced delivery. Although middle ear drug delivery devices may be useful for delivery of low molecular weight, stable, lipid soluble compounds like steroids, they will not be suitable for the delivery of the unstable macromolecules that ultimately will be the therapeutic compounds with greatest potential benefit.
4.3. Adaptation for chronic perfusion
The reciprocating drug delivery system should be easily adapted for chronic delivery of drugs to the inner ear. Even in an animal as small as the guinea pig, the pump is small enough to be mounted on the animal’s back and an injection valve system is easily miniaturizable with commercially available components. The animal would only need to be tethered by low voltage wire to the controller.
Ultimately, it should be possible to use this system for treatment of patients. The extension of microfabrication methods from integrated circuits to many other applications has spawned micro-electromechanical systems (MEMS) devices capable of reproducing the functions of conventional sensors and actuators at a fraction of the size [57,58]. The resulting miniaturization enables a complete system to be integrated into a device small enough to be implanted in the mastoid cavity. Complex automated dosing regimens could be programmed into the system or even designed to respond to sensor input of physiological measurements. Several companies are pursuing commercialization of microsystem-based drug delivery including Microchips (Bedford MA), Debiotech (Lausanne Switzerland), and ChipRx (Lexington KY). Moreover, technologies have emerged that may allow controlled release of drugs in dried or lyophilized form from discrete compartments [59-61]. These devices could allow high concentrations of drugs to be stored within the device and also could extend the long-term stability of emerging therapeutic compounds.
The technology of microfluidics enables precise control of liquid flow in the quantities needed to reproduce the performance of the system described here in a miniaturized form. The ability to regulate flow and manipulate tiny quantities of fluids has already had a major impact on ink jet dispensers and laboratory analysis systems in the form “lab-on-a-chip” techniques and microarrays (e.g., [62-64]). The success of electrophoresis in microchannels for genomics has been a spectacular example [65]. Investigators have capitalized on continuing innovations in microfabrication to develop individual microfluidic components such as valves and pumps as well as diverse microsensors that operate in microchannels or microwells [57,58,66-77] and several microscale active and passive flow regulators have been described [78-81]. Components such as these could be incorporated into an implantable device.
4.4. Clinical and research applications for this device
Research progress within the last decade is leading to new potential therapies for a broad spectrum of inner ear disorders. Therapies based on advances in cell and molecular biology will likely revolutionize the treatment of sensorineural hearing loss. Such treatments may require sequenced delivery of multiple and potentially unstable macromolecules for periods of months to years. It is likely that many of the newer treatments will have broad systemic effects if delivered by conventional techniques and may be highly specific macromolecules, such as those needed to direct cell repair. In either case, direct delivery of the drugs to the inner ear will minimize side effects, enable access to tissues of interest, and profoundly increase target specificity. As this rapidly expanding area of research continues to evolve, it is essential that researchers and clinicians begin to consider and develop mechanisms for the safe and sustained delivery of compounds to the inner ears of humans. While we have focused on cochlear applications, the approach will likely allow access of injected agents to the vestibular end organs. We have not assessed actions of agents we have injected on vestibular function. It seems likely that the approach can be modified to optimize access to other portions of the inner ear.
Application of the device for inner ear drug delivery is perhaps the most demanding of possible uses for the device. It should also be suited for local, timed, sequenced drug delivery in other inaccessible areas of the body, such as discrete regions of the central nervous system.
5. Conclusions
A reciprocating microfluidic system has been developed and demonstrated to provide safe and effective drug delivery into perilymph of the inner ear of the guinea pig. The system uses passive elements to control the reciprocating flow rates, and delivers drugs without cumulative fluid delivery into the cochlea. This infusion scheme eliminates the need for a second, effluent hole in the cochlea, reducing the probability of damage to the delicate structures of the inner ear. Monitoring frequency- (i.e. place-) dependent responses along the length of the cochlear partition provides a bioassay for drug distribution following administration and confirms that hearing is preserved throughout delivery with this approach. This device has been designed and demonstrated for intracochlear drug delivery, but should have broad applicability for therapies requiring precisely controlled localized delivery of drugs.
Acknowledgments
We thank Anthony A. Mikulec for helpful discussion. We are grateful for the technical assistance of Gerry Kujawa, Chris Scarpino, and Sarah Keller. This work was supported by grants from the NIDCD (R21 DC04983 (SGK); R01 DC00767 (WFS); RO1 DC03401-07 (MJM); P30 DC005209) and a Draper Extramural Clinical Research Award DLH-543157.
References
- 1.Raphael Y. Cochlear pathology, sensory cell death and regeneration. Br Med Bull. 2002;63:25–38. doi: 10.1093/bmb/63.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13(1):119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 3.Malgrange B, Rigo JM, Van de Water TR, Staecker H, Moonen G, Lefebvre PP. Growth factor therapy to the damaged inner ear: clinical prospects. Int J Pediatr Otorhinolaryngol. 1999;49(Suppl 1):S19–S25. doi: 10.1016/s0165-5876(99)00126-3. [DOI] [PubMed] [Google Scholar]
- 4.Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20(1):43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139(1–2):97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 6.Kopke R, Allen KA, Henderson D, Hoffer M, Frenz D, Van de Water T. A radical demise. Toxins and trauma share common pathways in hair cell death. Ann N Y Acad Sci. 1999;884:171–191. doi: 10.1111/j.1749-6632.1999.tb08641.x. [DOI] [PubMed] [Google Scholar]
- 7.Holley MC. Application of new biological approaches to stimulate sensory repair and protection. Br Med Bull. 2002;63:157–169. doi: 10.1093/bmb/63.1.157. [DOI] [PubMed] [Google Scholar]
- 8.Gao WQ. Therapeutic potential of neurotrophins for treatment of hearing loss. Mol Neurobiol. 1998;17(1–3):17–31. doi: 10.1007/BF02802022. [DOI] [PubMed] [Google Scholar]
- 9.Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- 10.Seidman MD, Van De Water TR. Pharmacologic manipulation of the labyrinth with novel and traditional agents delivered to the inner ear. Ear Nose Throat J. 2003;82(4):276–280. 282–273, 287–278. passim. [PubMed] [Google Scholar]
- 11.Ryan AF, Pak K, Low W, Battaglia A, Mullen L, Harris JP, Keithley EM. Immunological damage to the inner ear: current and future therapeutic strategies. Adv Oto-Rhino-Laryngol. 2002;59:66–74. doi: 10.1159/000059242. [DOI] [PubMed] [Google Scholar]
- 12.Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99(3):1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JM, Miller AL, Yamagata T, Bredberg G, Altschuler RA. Protection and regrowth of the auditory nerve after deafness: neurotrophins, antioxidants and depolarization are effective in vivo. Audiol Neuro-Otol. 2002;7(3):175–179. doi: 10.1159/000058306. [DOI] [PubMed] [Google Scholar]
- 15.Petit C. Genes responsible for human hereditary deafness: symphony of a thousand. Nat Genet. 1996;14(4):385–391. doi: 10.1038/ng1296-385. [DOI] [PubMed] [Google Scholar]
- 16.Steel KP, Kros CJ. A genetic approach to understanding auditory function. Nat Genet. 2001;27:143–149. doi: 10.1038/84758. [DOI] [PubMed] [Google Scholar]
- 17.Lalwani AK, Jero J, Mhatre AN. Current issues in cochlear gene transfer. Audiol Neuro-Otol. 2002;7(3):146–151. doi: 10.1159/000058300. [DOI] [PubMed] [Google Scholar]
- 18.Luebke AE, Steiger JD, Hodges BL, Amalfitano A. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther. 2001;8(10):789–794. doi: 10.1038/sj.gt.3301445. [DOI] [PubMed] [Google Scholar]
- 19.Inamura N, Salt AN. Permeability changes of the blood–labyrinth barrier measured in vivo during experimental treatments. Hear Res. 1992;61(1–2):12–18. doi: 10.1016/0378-5955(92)90030-q. [DOI] [PubMed] [Google Scholar]
- 20.Juhn SK, Rybak LP. Labyrinthine barriers and cochlear homeostasis. Acta Oto-Laryngol. 1981;91(5–6):529–534. doi: 10.3109/00016488109138538. [DOI] [PubMed] [Google Scholar]
- 21.Tonndorf J, Duvall AJ, Reneau JP. Permeability of intracochlear membranes to various vital stains. Ann Otol Rhinol Laryngol. 1962;71:801–841. doi: 10.1177/000348946207100317. [DOI] [PubMed] [Google Scholar]
- 22.Salt AN, Henson MM, Gewalt SL, Keating AW, DeMott JE, Henson OW., Jr Detection and quantification of endolymphatic hydrops in the guinea pig cochlea by magnetic resonance microscopy. Hear Res. 1995;88(1–2):79–86. doi: 10.1016/0378-5955(95)00103-b. [DOI] [PubMed] [Google Scholar]
- 23.Shinomori Y, Spack DS, Jones DD, Kimura RS. Volumetric and dimensional analysis of the guinea pig inner ear. Ann Otol Rhinol Laryngol. 2001;110(1):91–98. doi: 10.1177/000348940111000117. [DOI] [PubMed] [Google Scholar]
- 24.Buckingham RA, Valvassori GE. Inner ear fluid volumes and the resolving power of magnetic resonance imaging: can it differentiate endolymphatic structures? Ann Otol Rhinol Laryngol. 2001;110(2):113–117. doi: 10.1177/000348940111000204. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol. 1997;381(2):188–202. [PubMed] [Google Scholar]
- 26.Stypulkowski PH. Mechanisms of salicylate ototoxicity. Hear Res. 1990;46(1–2):113–145. doi: 10.1016/0378-5955(90)90144-e. [DOI] [PubMed] [Google Scholar]
- 27.Kujawa SG, Fallon M, Bobbin RP. Intracochlear salicylate reduces low-intensity acoustic and cochlear microphonic distortion products. Hear Res. 1992;64(1):73–80. doi: 10.1016/0378-5955(92)90169-n. [DOI] [PubMed] [Google Scholar]
- 28.Littman T, Bobbin RP, Fallon M, Puel JL. The quinoxalinediones DNQX, CNQX and two related congeners suppress hair cell-to-auditory nerve transmission. Hear Res. 1989;40(1–2):45–53. doi: 10.1016/0378-5955(89)90098-1. [DOI] [PubMed] [Google Scholar]
- 29.Kujawa SG, Liberman MC. Conditioning-related protection from acoustic injury: effects of chronic deefferentation and sham surgery. J Neurophysiol. 1997;78(6):3095–3106. doi: 10.1152/jn.1997.78.6.3095. [DOI] [PubMed] [Google Scholar]
- 30.Beentjes B. On the pressure of the endolymphatic, the perilymphatic, and the cerebrospinal fluid, with data on the endolymphatic membranes: experiments on cats and guinea pigs. Universiteit van Amsterdam. 1970:56. [Google Scholar]
- 31.Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11(2):82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fex J. In: Hearing Mechanisms in Vertebrates. DeReuck AVS, Knight J, editors. Little, Brown & Co.; Boston: 1968. pp. 169–181. [Google Scholar]
- 33.Konishi T. Action of tubocurarine and atropine on the crossed olivocochlear bundles. Acta Oto-Laryngol. 1972;74(4):252–264. doi: 10.3109/00016487209128447. [DOI] [PubMed] [Google Scholar]
- 34.Galley N, Klinke R, Oertel W, Pause M, Storch WH. The effect of intracochlearly administered acetylcholine-blocking agents on the efferent synapses of the cochlea. Brain Res. 1973;64:55–63. doi: 10.1016/0006-8993(73)90170-4. [DOI] [PubMed] [Google Scholar]
- 35.Bobbin RP, Konishi T. Acetylcholine mimics crossed olivocochlear bundle stimulation. Nat New Biol. 1971;231(24):222–223. doi: 10.1038/newbio231222a0. [DOI] [PubMed] [Google Scholar]
- 36.Nuttall AL, LaRouere MJ, Lawrence M. Acute perilymphatic perfusion of the guinea pig cochlea. Hear Res. 1982;6(2):207–221. doi: 10.1016/0378-5955(82)90055-7. [DOI] [PubMed] [Google Scholar]
- 37.Puel JL, Pujol R, Tribillac F, Ladrech S, Eybalin M. Excitatory amino acid antagonists protect cochlear auditory neurons from excitotoxicity. J Comp Neurol. 1994;341(2):241–256. doi: 10.1002/cne.903410209. [DOI] [PubMed] [Google Scholar]
- 38.Salt AN, Stopp PE. The effect of cerebrospinal fluid pressure on perilymphatic flow in the opened cochlea. Acta Oto-Laryngol. 1979;88(3–4):198–202. doi: 10.3109/00016487909137160. [DOI] [PubMed] [Google Scholar]
- 39.Sewell WF, Norris CH, Tachibana M, Guth PS. Detection of an auditory nerve-activating substance. Science. 1978;202(4370):910–912. doi: 10.1126/science.30998. [DOI] [PubMed] [Google Scholar]
- 40.Kujawa SG, Erostegui C, Fallon M, Crist J, Bobbin RP. Effects of adenosine 5′-triphosphate and related agonists on cochlear function. Hear Res. 1994;76(1–2):87–100. doi: 10.1016/0378-5955(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 41.Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. A nicotinic-like receptor mediates suppression of distortion product otoacoustic emissions by contralateral sound. Hear Res. 1994;74(1–2):122–134. doi: 10.1016/0378-5955(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 42.Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of efferent stimulation on cochlear potentials in the guinea pig. J Neurosci. 1995;15(5 Pt 1):3667–3678. doi: 10.1523/JNEUROSCI.15-05-03667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kujawa SG, Fallon M, Bobbin RP. ATP antagonists cibacron blue, basilen blue and suramin alter sound-evoked responses of the cochlea and auditory nerve. Hear Res. 1994;78(2):181–188. doi: 10.1016/0378-5955(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 44.Erostegui C, Norris CH, Bobbin RP. In vitro pharmacologic characterization of a cholinergic receptor on outer hair cells. Hear Res. 1994;74(1–2):135–147. doi: 10.1016/0378-5955(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 45.Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70(2):167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- 46.Kingma GG, Miller JM, Myers MW. Chronic drug infusion into the scala tympani of the guinea pig cochlea. J Neurosci Methods. 1992;45(1–2):127–134. doi: 10.1016/0165-0270(92)90050-n. [DOI] [PubMed] [Google Scholar]
- 47.Prieskorn DM, Miller JM. Technical report: chronic and acute intracochlear infusion in rodents. Hear Res. 2000;140(1–2):212–215. doi: 10.1016/s0378-5955(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 48.Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15(4–5):631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 49.Stover T, Yagi M, Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7(5):377–383. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- 50.Carvalho GJ, Lalwani AK. The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am J Otol. 1999;20(1):87–90. [PubMed] [Google Scholar]
- 51.McCabe BF. Autoimmune inner ear disease: therapy. Am J Otol. 1989;10(3):196–197. [PubMed] [Google Scholar]
- 52.Moskowitz D, Lee KJ, Smith HW. Steroid use in idiopathic sudden sensorineural hearing loss. Laryngoscope. 1984;94(5 Pt 1):664–666. [PubMed] [Google Scholar]
- 53.Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106(12):772–776. doi: 10.1001/archotol.1980.00790360050013. [DOI] [PubMed] [Google Scholar]
- 54.Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999;109(7 Pt 2):1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- 55.Plontke S, Zenner HP. Pharmacokinetic considerations in intratympanic drug delivery to the inner ear. Acta Oto-Rhino-Laryngol Belg. 2002;56(4):369–370. [PubMed] [Google Scholar]
- 56.Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear Res. 2001;154(1–2):88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 57.Madou M. Fundamentals of Microfabrication. CRC Press; Boca Raton, FL: 2002. [Google Scholar]
- 58.Kovacs GTA. Micromachined Transducers Sourcebook. McGraw-Hill; 1998. [Google Scholar]
- 59.Langer R. Drugs on target. Science. 2001;293:58–59. doi: 10.1126/science.1063273. [DOI] [PubMed] [Google Scholar]
- 60.Santini JT, Cima MJ, Langer R. A controlled release microchip. Nature. 1999;397:335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 61.Madou MJ, He K-Q. Exploitation of a novel artificial muscle for controlled drug delivery. Polym Mater Sci Eng. 2000;83:495–497. [Google Scholar]
- 62.Duffy DC, Gillis HL, Lin J, Sheppard NFJ, Kellogg GJ. Microfabricated centrifugal microfluidic systems: characterization and multiple enzymatic assays. Anal Chem. 1999;71(20):4669–4678. [Google Scholar]
- 63.Schena M. Microarray Biochip Technology. Eaton Publishing; Natick, MA: 2000. [Google Scholar]
- 64.Ekins RP. Ligand assays: from electrophoresis to miniaturized microarrays. Clin Chem. 1998;44:2015–2030. [PubMed] [Google Scholar]
- 65.Jacobson SC, Hergenroder R, Koutny LB, Ramsey JM. High-speed separations on a microchip. Anal Chem. 1994;66:1114–1118. [Google Scholar]
- 66.Mescher MJ, Dube CE, Varghese M, Fiering JO. Surface mount microfluidic flow regulator on a polymer substrate. MicroTAS; Squaw Valley CA. to be presented Oct 2003. [Google Scholar]
- 67.Nguyen N-T, Wereley ST. Fundamentals and Applications of Microfluidics. Artech House; Norwood, MA: 2002. [Google Scholar]
- 68.Zengerle R. A bidirectional silicon micropump. Sens Actuators, A. 1995;50:81–86. [Google Scholar]
- 69.Yun KS, Cho IJ, Bu JU, Kim GH, Jeon YS, Kim CJ, Yoon E. A micropump driven by continuous electrowetting actuation for low voltage and low power operations. MEMS 2001, 14th International Conf. on Micro Electro Mechanical Systems; Interlaken, Switzerland. 2001; pp. 487–490. [Google Scholar]
- 70.Maillefer D, Gamper S, Frehner B, Balmer P, Van Lintel H, Renaud P. A high-performance silicon micropump for disposable drug delivery systems. 14th IEEE International Conference on Micro Electro Mechanical Systems; IEEE Interlaken, Switzerland. 2001; pp. 413–417. [Google Scholar]
- 71.Bohm S. A plastic micropump constructed with conventional techniques and materials. Sens Actuators, A. 1999;77(3):223–228. [Google Scholar]
- 72.Tsai JH, Lin L. A thermal-bubble-actuated micronozzlediffuser pump. J Microelectromech Syst. 2002;11(6):665–671. [Google Scholar]
- 73.Wego A, Pagel L. A self-filling micropump based on PCB technology. Sens Actuators, A. 2001;88:220–226. [Google Scholar]
- 74.Van Lintel HTG. A piezoelectric micropump based on micromachining of silicon. Sens Actuators. 1998;15:153–167. [Google Scholar]
- 75.Goettsche T, Kohnle J, Willmann M, Ernst H, Messner S, Steger R, Storz M, Lang W, Zengerle R, Sandmaier H. Novel approaches to microfluidic components in high-end medical applications. Transducers, Solid-state Sensors, Actuators and Microsystems, 12th Int. Conf. on IEEE; 2003; pp. 623–626. [Google Scholar]
- 76.Su YC, Lin L, Pisano AP. A water-powered micro drug delivery system. Solid-State Sensor, Actuator and Microsystems Workshop; Hilton Head Island. 2002. [Google Scholar]
- 77.Capanu M, Boyd JG, Hesketh PJ. Design, fabrication, and testing of a bistable electromagnetically actuated microvalve. J Microelectromech Syst. 2000;9:181–189. [Google Scholar]
- 78.Yu Q, Bauer JSMJM, Beebe DJ. Responsive biomimetic hydrogel valve for microfluidics. Appl Phys Lett. 2001;78:2589–2591. [Google Scholar]
- 79.Fitch JS. Pressure-based mass-flow control using thermopneumatically-actuated microvalves. Proceedings Sensors and Actuators Workshop; Cleveland. 1998; pp. 162–165. [Google Scholar]
- 80.Cousseau P. Improved micro-flow regulator for drug delivery systems. Proceedings Micro Electro Mechanical Systems; 2001; 2001. pp. 527–530. [Google Scholar]
- 81.Cabuz E. MEMS-based flow controller for flow cytometry. Proceedings Solid State Sensor, Actuator, and Microsystems Workshop; Hilton Head (SC). 2002; pp. 110–111. [Google Scholar]


