Abstract
Previously, we reported that lateral hypothalamic (LH) orexin neurons are stimulated in proportion to the preference shown for reward-associated cues during conditioned place preference (CPP) testing. Here, we examine for the first time the role of these neurons in the acquisition of morphine CPP. Results show that LH orexin neurons, but not those in the perifornical area (PFA), are stimulated during conditioning when morphine is given in a novel drug-paired environment (CPP compartment) but not when given in the home cage, nor when saline was given in the CPP environment. Furthermore, bilateral excitotoxic lesions of the LH orexin area completely blocked the acquisition of morphine CPP. Lesions that spared LH orexin neurons had no effect. Orexin neurons in the LH project to the ventral tegmental area (VTA), an area important in the acquisition of morphine CPP. Therefore, we investigated the importance of the LH orexin connection to the VTA in the acquisition of a morphine CPP using a disconnection technique involving a unilateral excitotoxic lesion of LH orexin neurons and contralateral blockade of VTA orexin receptors. Results indicated that a unilateral LH orexin lesion together with a microinjection of the orexin A antagonist (SB 334867) into the contralateral VTA prior to each morphine-pairing session was sufficient to block the development of a morphine CPP. Either of these treatments by themselves was not sufficient to block CPP development. These results demonstrate the importance of LH orexin neurons and their projections to the VTA in the formation of associations between environmental cues and drug reward.
Keywords: Morphine, Morphine Place Conditioning, Lateral Hypothalamus, Orexin, Hypocreatin, Ventral Tegmental Area, Lesion
1.Introduction
Recently a number of studies have revealed a novel and important role for the orexin/hypocretin neuronal system in reward processing and addiction [3,4,11,15,18,21,25]. The orexin neurons are located primarily in the lateral (LH) and perifornical (PFA) regions of the posterior hypothalamus [7,30], and have extensive projections throughout the central nervous system [29]. Previous studies indicated that the orexin neuropeptide transmitters were associated with feeding, arousal and the maintenance of waking [6,22,30,32,35]. Orexin neurons, however, heavily innervate both the dopamine (DA)-rich ventral tegmental area (VTA), as well as the nucleus accumbens (NAc) [10], structures that drive behaviors motivated by either food or drug rewards. Moreover, orexin receptors are expressed at high levels in both of these areas [20,24,33].
Recently, we showed that orexin neurons in the LH are activated by cues associated with rewards such as food or drugs, and that exogenous stimulation of LH orexin neurons can reinstate extinguished drug-seeking behavior in rodents [15]. The LH has long been implicated in homeostatic regulation, and lesion and intracranial self-stimulation studies have revealed an important role for this area in feeding, arousal, and reward [1,27]. The role of orexin in the reinstatement of drug seeking may be mediated in part via the VTA, because we found that orexin administration directly into the VTA also reinstated an extinguished drug preference [15]. In addition, Narita and colleagues [25] recently showed that infusions of an orexin-A antagonist into the VTA prevented the acquisition of a conditioned morphine place preference, indicating that orexin release in the VTA is necessary for learning the association between environmental cues and morphine reward.
Recently, we proposed that there is a dichotomy in function between orexin neurons located in the PFA region of the hypothalamus, and those located in the LH [12,15]. In support of this idea, we found that LH orexin neurons respond to cues associated with food and drug reward but do not respond to footshock, whereas PFA orexin neurons respond to footshock but not to reward-associated cues [15]. In the present experiments we sought to determine whether LH orexin neurons, and their projections to the VTA, are essential for learning a CPP for morphine. We first measured the activation of LH orexin neurons during acquisition of a morphine CPP. Next, we established the effect of lesioning LH orexin neurons on learning a morphine CPP. Finally, we used a contralateral disconnection approach to test the role of LH orexin projections to the VTA in learning a morphine CPP.
2. Materials and Methods
2.1 Subjects
Male Sprague-Dawley rats (250-300 g; Harlan, Indianapolis, IN, USA; n=60) were used in all experiments. Rats were group-housed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and all efforts were made to minimize the number of animals used and their suffering. Animals were maintained on a 12-h light/dark cycle with food and water available ad libitum. All animal procedures were also approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
2.2 Drugs
Morphine sulfate powder (National Institute on Drug Abuse, Baltimore, MD, USA), was dissolved in sterile saline and administered via i.p. injection. Ibotenic acid (Acros Organics, Geel, Belgium) was dissolved in 10% 2N ammonium hydroxide and sterile water. N-Methyl-D-aspartic acid (NMDA) (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile water. The orexin antagonist SB 334867 (Tocris, Ellisville, MO, USA) was dissolved in artificial cerebral spinal fluid. The doses of ibotenic acid and NMDA were based on publications using similar neurotoxic microinjections [5,19,23,26]. The dose of SB 334867 was based on published reports using similar microinjections [25,31].
2.3 CPP procedure
As previously described [16], conditioning and testing occurred in a Plexiglas apparatus that consisted of two distinct compartments separated by a Plexiglas divider. Each compartment was equipped with photo cells to automatically log time and activity (MED Associates, Inc.). One compartment had a grid floor with black walls, and the other compartment had a mesh floor with black and white stripes on the walls. On the first day, all rats were allowed to freely explore all of the apparatus for 15 minutes, and the amount of time spent in each compartment was recorded. None of the animals had an initial bias for either compartment (time difference < 100 sec), and each was randomly assigned to a compartment for morphine conditioning in a balanced design. Conditioning began the day after this preconditioning day. On each of the next 3 days, rats were given injections of either saline or morphine (8 mg/kg, i.p.) and were confined to the assigned compartment for 30 minutes. Morphine and saline sessions were alternated between the morning and afternoon with a four hour interval between sessions. Rats given morphine in the morning were given saline in the opposite chamber in the afternoon, and on subsequent days received saline in the morning and morphine in the afternoon (Day 1 for all groups always began with the saline session followed by the morphine session).
2.4 Double label immunohistochemistry
Two hours after conditioning, or after the morphine or saline injections in animals that were not conditioned (see Experiment 1 methods below), rats were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/Kg, i.p.) and perfused transcardially with paraformaldehyde as previously described [14]. Brain sections were first processed for Fosimmunostaining with nickel ammonium sulfate intensification of DAB and then processed for orexin as previously described [15] (orexin A antibody, Santa Cruz Biotechnology, Inc., 1:1000; biotinylated secondary, 1:500). Slices were mounted on glass slides, dehydrated in alcohol and cover slipped. Double-labeled cells were readily identified because orexin stained the cytoplasm brown and Fos immunoreactive nuclei were black. For counts, two sections from each animal in each group were chosen at the same levels with equivalent numbers of orexin-positive neurons. Fos-positive cells were quantified using Openlab image processing software (Improvision, Ltd.; Coventry England) on a MacIntosh computer that was linked to a microscope and digital camera. Color images of the LH and PFA were taken and saved to disc. The number of orexin+ neurons, and Fos+/orexin+ doubly labeled neurons, were counted with a point-counter tool by a blind observer on the saved image and averaged across sections for each animal. This tool simultaneously marked and counted each cell so that no cells could be counted twice and the total number of cells counted was available. All orexin-labeled neurons lateral to the fornix were considered to be in the lateral hypothalamus. All orexin-labeled neurons located above and below the fornix, and up to 0.4 mm medial to the fornix, were considered to be in the PFA.
2.5 Experiment 1
Our standard CPP training procedure (as described above) was used for all animals given injections in the CPP boxes [16]. Rats were given a single injection of morphine (n=4) or saline (n=4) and confined to one compartment for 30 min. Compartments were randomly assigned and each compartment was counterbalanced between groups. For the home cage group (n=4), animals were given the same alternating injections of morphine and saline as the CPP groups but injections were made in a separate procedure room, and animals were subsequently confined to their home cage for 30 min. For animals sacrificed on Day 1 of conditioning, each rat was perfused 2 h after morphine or saline conditioning. For animals sacrificed on Day 3 of conditioning, the first and second conditioning days were similar to our standard CPP procedure. On Day 3 of conditioning, rats were given either the third morphine (n=4) or saline (n=4) injection in one compartment and sacrificed 2 h after their 30 min confinement.
2.6 Experiment 2
Rats were anesthetized with sodium pentobarbital (50 mg/Kg, i.p.) and a glass micropipette (20 um tip) was lowered into the LH orexin field (n=7; AP −3.1, ML +/− 1.7, DV −8.1) [28] or into areas surrounding the LH orexin field (n=13). Rats received bilateral pressure injections of ibotenic acid (10 μg/μl, 600 nl) for 15 min. Animals were allowed one week to recover from surgery before CPP training began.
2.7 Experiment 3
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and implanted with a unilateral guide cannula aimed 1.5 mm above the VTA (AP −5.3, ML +2.5, DV −7.5; Paxinos and Watson, 1998). The cannula (26 gauge) was angled 100 (dorsolateral to ventromedial). During the same surgery, a glass micropipette (20 um tip) was lowered into the contralateral LH orexin field (n=7; AP −3.1, ML +/− 1.7, DV −8.1) (Paxinos and Watson, 1998) to microinject NMDA. In this experiment we used NMDA as opposed to ibotenic acid because a preliminary comparison of the two methods revealed that the NMDA lesions were slightly less toxic to non-orexin cells. Furthermore, a recent publication revealed that NMDA was more selectively toxic to orexin than to melanin-concentrating hormone (MCH) neurons [17]. MCH cells are located in the same region as the orexin cells. A pressure injection of NMDA (10 μg/μl, 600 nl) or vehicle was given for 15 min. The sides chosen for VTA cannula and LH neurotoxin lesion were alternated in a Latin square design.
All animals were given one week to recover from surgery before place conditioning training. During conditioning, an inner injection cannula (30 gauge) was inserted 1.5 mm below the guide cannula into the VTA injection site. Rats with unilateral LH lesions received a contralateral intra-VTA microinjection of the orexin A antagonist, SB 334867 (2.4 nmole/ 0.3 μl), 5 min prior to each morphine conditioning trial (n=9). In another group of unilaterally LH lesioned animals, similar injections of SB 334867 were made outside the contralateral VTA (n=3) 5 min prior to each morphine trial. To test the effects of unilateral injections of SB 334867 alone on conditioning, some animals received either a vehicle injection in the LH (n=6), or an NMDA injection outside the LH (n=3), which spared orexin neurons, and were then administered a contralateral microinjection of SB 334867 on conditioning days.
2.8 Verification of lesions in Experiments 2 and 3
After CPP testing, animals were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/kg, i.p.). Rats were perfused with saline (0.9%) followed by perfusion with 4% paraformaldehyde. Brains were then collected to allow subsequent immunohistochemistry and histological confirmation. Forty-micron-thick sections were cut through the LH and stained for orexin A (as described above). Six sections at the level of the lesion were chosen from each animal, mounted on glass slides, dehydrated in alcohols and coverslipped. As described for Experiment 1, color photographs of the LH and PFA were taken and the numbers of bilateral orexin neurons were counted for all 6 sections for each animal by a blind observer.
2.9 Data analyses
In Experiment 1, the percentages of orexin+ neurons that were also Fos+ (doubly labeled) were compared between groups using a 2-way ANOVA (day X treatment). In Experiments 2 and 3, place conditioning data were analyzed by calculating the time spent in the reward-paired compartment minus the time spent in the same compartment prior to conditioning. The resulting difference score was compared between groups using either an analysis of variance (ANOVA) or a t-test. For Experiment 2 (bilateral lesions), the number of surviving orexin neurons was counted and averaged across the right and left sides to yield a single number for each section. For Experiment 3, the number of surviving orexin neurons were counted and compared between the lesioned versus non-lesioned sides. Counts of surviving orexin neurons post-lesion were compared between groups (bilateral lesion) or within subjects (unilateral lesion) using an ANOVA or a t-test. Where necessary, post-hoc analysis was carried out with a Newman-Keuls test.
3. Results
3.1 Experiment 1
Figure 1 shows the results of Experiment 1. On the first day of conditioning a significant increase in Fos activation was seen in LH orexin neurons only in animals given morphine reward in the CPP environment (Figure 1a). A two-way ANOVA revealed a significant Group effect (F(2,18)=37, P<.01) and a significant Days effect (F(1,18)=20, P<.01). Follow up analyses revealed that the groups given morphine in the CPP box were significantly different from the groups given morphine in the home cage, and from the groups given saline in the CPP box on both Days 1 and 3 of CPP acquisition (P<.01).
Figure 1.
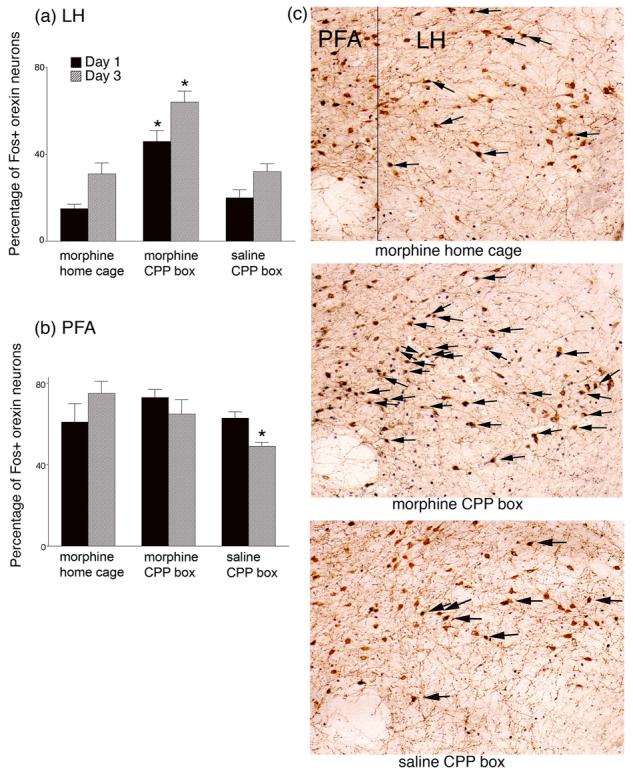
Activation (as indicated by Fos immunoreactivity) of orexin neurons in the lateral hypothalamus (LH) (a) and perifornical area (PFA) (b) following morphine administration in the home cage or conditioned place preference box (CPP), or following saline in the CPP box, on the first and third conditioning days. All animals in each group received a single injection of morphine (10 mg/kg) or saline and were exposed to the CPP environment, or returned to their home cage, for 30 min. Animals were perfused 2 hours after drug or saline conditioning, and brains were processed for orexin and Fos immunohistochemistry. Standard daily alternating injections of morphine and saline were given to all Day 3 animals on Days 1 and 2 of the procedure. *significantly different from other groups P<.01. (c) Photomicrographs of the lateral hypothalamus taken from representative animals on Day 1 of drug or saline exposure. Arrows indicate orexin positive neurons that also were Fos positive. In all cases medial is on the left. f=fornix, LH=lateral hypothalamus.
In the PFA (Fig. 1b) there was a small but significant Group effect (F(2,18)=3, P<.05) and a Group X Day interaction (F(2,18)=3, P<.05). Follow-up analyses revealed a significant decline in Fos activation in PFA orexin neurons on Day 3 of saline administration in the CPP box (P<.05) accounting for both the Group effect and the Group X Day interaction. This most likely reflects habituation to the saline injection and a generalized decrease in arousal in this group. For the groups given morphine injections in either the CPP box or the home cage, no differences in Fos activation were seen in the PFA orexin neurons on either day of acquisition. This indicates that the PFA orexin neurons respond to the arousing effects of a morphine injection but unlike the LH orexin neurons, they did not differentiate between the context in which the injection was given.
Fos activation in non-orexin neurons in the LH was low in number <10 neurons per slice) and variable. Previously we have found that Fos activation in non-orexin positive neurons was inconsistent and did not correlate with preference behavior [15].
3.2 Experiment 2
Figure 2a shows the results of the bilateral LH orexin lesion on CPP acquisition. After analysis of the damage to LH orexin neurons, animals were divided into two groups. Animals in which both sides of the LH orexin field were severely damaged (losses of greater than 50% of the mean of control animals, Figure 2b; n=7) were considered to be lesioned. Animals that had unilateral lesions in which only one side of the LH orexin field was damaged (e.g., the contralateral ibotenic acid injection was dorsal or ventral to the LH orexin field; n=4) or in which the LH orexin neurons were spared on both sides (n=5) were considered control-lesioned. In the 5 control animals in which no LH orexin neurons were damaged, the ibotenic acid injections were either too rostral (n=1, bregma location rostral to −2.3 mm), too caudal (n=3, bregma locations caudal to −3.6 mm) or too medial (n=1, injection was medial to the fornix causing damage to the PFA orexin neurons) to affect the LH orexin neurons.
Figure 2.
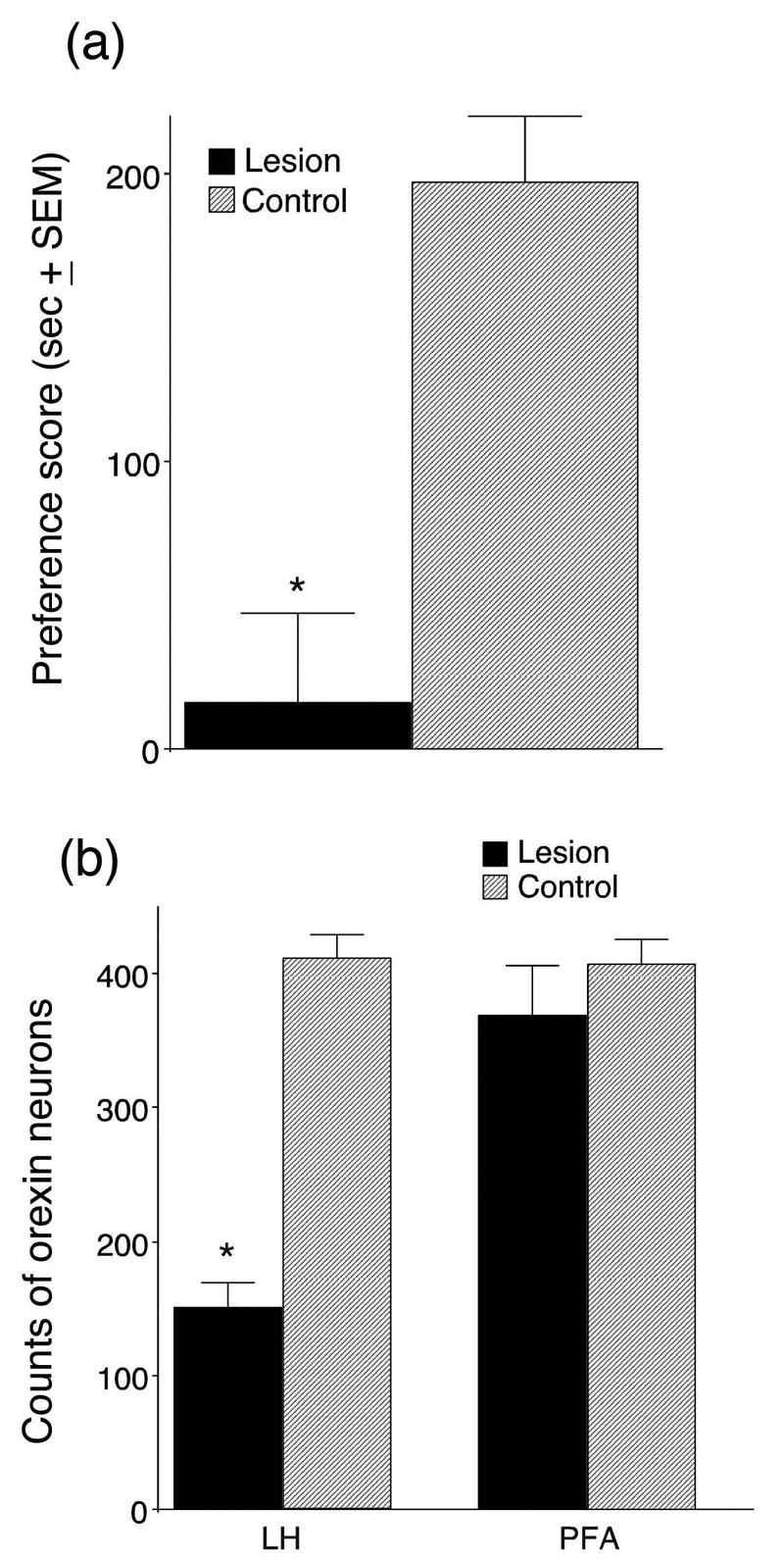
Conditioned place preference scores (a) and orexin-positive neuronal cell counts (b) for animals given bilateral ibotenic acid microinjections in the lateral hypothalamus (LH). (a) Preference scores were calculated by subtracting the time spent in the morphine-paired chamber on the preconditioning day from the time spent in that chamber on the postconditioning (test) day. Scores represent group means ± standard errors. (b) Counts of orexin-positive neurons in LH or perifornical area (PFA) from 6 adjacent 40 um-thick sections in each animal at the level of the neurotoxin injection. Control animals were given similar ibotenic acid injections that were either dorsal or caudal to the orexin cell field. Sections from control animals were chosen to match the same anterior/posterior levels as the animals with orexin neuron lesions (*P<.01, lesioned group n=7, control n=9).
In the LH orexin-lesioned group no animal had a preference score above 100 sec. A within-group comparison of preference scores pre- and post-conditioning was not significant (t(5)=−0.7, P=.42). In contrast, in the control-lesioned group all animals had preference scores above 120 sec (including the subject with PFA lesions) and a within-group comparison of pre- versus post-conditioning preference scores showed a significant difference (t(7)=12, P<.01). In addition, the morphine preference exhibited by the four control rats with unilateral orexin neuron lesions was not significantly different from that for the animals in which no orexin neurons were damaged (t(7)=0.6, P=.54). Regardless of the location of the ibotenic acid injections outside of the LH, animals without substantial LH orexin neuron damage (lesioned-control) showed low variability in their preference scores (SEM = 23 sec). A between-group comparison of preference scores for the lesioned and control-lesioned groups revealed a significant group difference with the control group showing significantly greater preference scores (t(14)=−5, P<.01).
Animals with bilateral lesions of LH orexin neurons that failed to develop a CPP response also had significantly fewer orexin neurons in the LH than control-lesioned animals (t(14)=−10, P<.01, Figure 2b). The locations of ibotenic acid lesions in the LH orexin lesion group were similar among group members and, although some animals had a small amount of damage in the PFA region, most of the lesion was located lateral to the fornix (Figure 3). The LH-lesion and control-lesioned groups did not differ in terms of the number of surviving PFA orexin neurons (t(14)=−0.9, P=.35, Figure 2b) nor were they different in terms of their locomotor responses to morphine during conditioning (t(14)=0.6, P=.54) or their locomotor responses on the CPP test day (t(14)=−0.7, P=.44).
Figure 3.
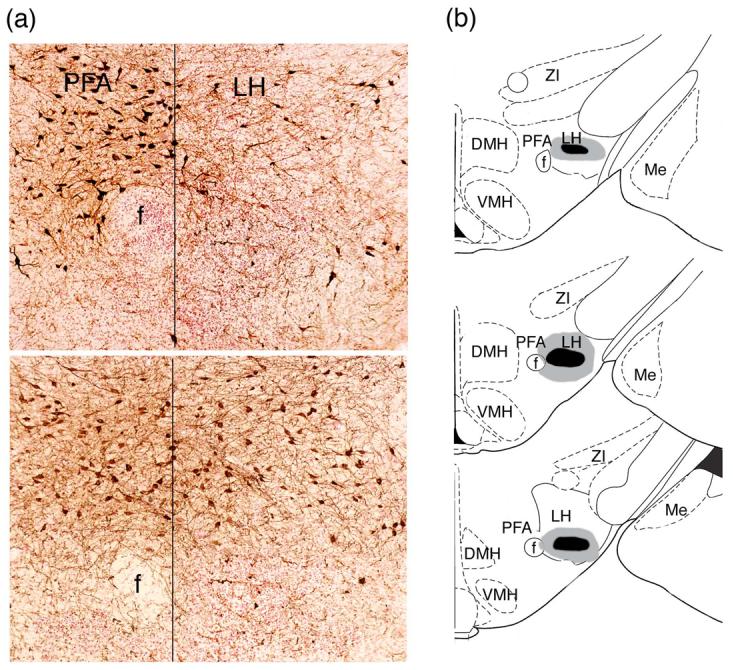
Photomicrographs (a) and diagram (b) illustrating the ibotenic acid lesions of the lateral hypothalamus. (a) Photomicrographs of an effective ibotenic acid lesion from an animal that failed to learn morphine CPP (top photograph) and an ineffective ventral control lesion from an animal that developed a morphine CPP (bottom photograph). (b) Diagram showing the extent of the effective ibotenic acid LH orexin lesions. The gray shaded area indicates the largest extent of the lesions and the black area indicates the smallest. Drawings were adapted from Paxinos and Watson 1998. ZI=zona incerta, DMH=dorsomedial hypothalamus, LH=lateral hypothalamus, PFA perifornical area, Me= medial amygdala nuc., VMH=ventomedial hypothalamus, Arc=arcuate nuc., f=fornix.
3.3 Experiment 3
The results of Experiment 3 are shown in Figure 4. Animals with a unilateral lesion of LH orexin neurons coupled with an injection of the orexin A antagonist SB 334867 into the contralateral VTA during the morphine conditioning trials all failed to develop preference scores above 50 sec. A within-group comparison of pre- versus post-conditioning preference was not significant (t(7)=.24, P=.59).
Figure 4.
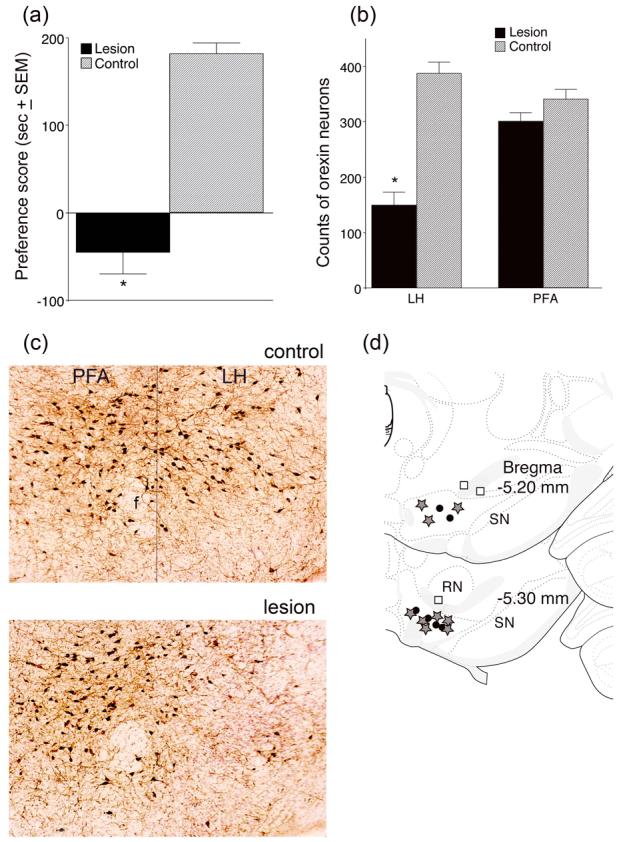
Conditioned place preference (CPP) scores (a) and orexin neuronal cell counts (b) for animals given unilateral NMDA injections in the LH and microinjections of SB 334867 in the contralateral VTA during CPP training. (a) Preference scores were calculated by subtracting the time spent in the morphine-paired chamber during the preconditioning day from the time spent in that chamber on the test day (i.e., post-conditioning). Control animals received vehicle instead of NMDA in the LH and received the same SB injections in the contralateral VTA. Scores represent group means ± standard errors. (b) Neuronal cell counts of surviving orexin neurons from 6 adjacent 40 um-thick sections at the level of the neurotoxin injection from animals with effective lesions. Control refers to the number of orexin neurons found on the non-lesioned side in the same slices (*P<.01, n=9). (c) Photomicrographs of an effective NMDA lesion from one experimental animal. f=fornix, PFA=perifornical area, LH=lateral hypothalamus (d) Diagram of VTA injection sites. The grey stars indicate effective sites of SB 334867 injection when combined with effective LH orexin lesion, black circles indicate SB injection sites in animals without LH lesions, and the white squares indicate ineffective SB injection sites in animals with LH orexin lesions. SN=substantia nigra, RN=red nucleus. Drawings were adapted from Paxinos and Watson 1998.
Two control groups were used in this experiment: One was given a vehicle injection in the LH (sham lesion) coupled with a microinjection of SB 334867 in the contralateral VTA during morphine conditioning trials (n=6), and the second either had no lesion of LH orexin neurons (the NMDA lesion fell outside the range of the LH orexin neurons, n=3) or the SB 334867 injection occurred outside the VTA (n=3). The CPP scores for animals in these control groups did not differ from each other (t(10)=0.4, P=.67) and therefore their data were combined to form a single control group. All animals in this control group developed preference scores above 120 sec and a comparison of pre- versus post-conditioning preferences revealed a significant within group difference (t(10)=−8, P<.01). The control and experimental groups differed significantly in CPP scores (t(19)=−8, P<.01, Figure 4a) but were not different in terms of their locomotor response to morphine during conditioning (t(19)=0.4, P=.66) or their locomotor response on the CPP test day (t(19)=0.4, P=.69). For the 9 animals in the experimental group that had an NMDA injection in the LH, the number of surviving orexin neurons on the injected side was compared to the number of neurons on the non-injected side. This analysis revealed a significant decrease in the number of orexin neurons between the two hemispheres for the LH (t(16)=−7, P<.01) but not for the PFA (t(16)=−1.6, P=.12, Figure 4b). Figure 4c illustrates the differences seen between the control and lesioned LH orexin neurons in one experimental animal. All animals in the experimental group had SB 334867 injections located in the VTA (Figure 4d). Figure 5 illustrates the greatest extent of the effective NMDA lesions.
Figure 5.
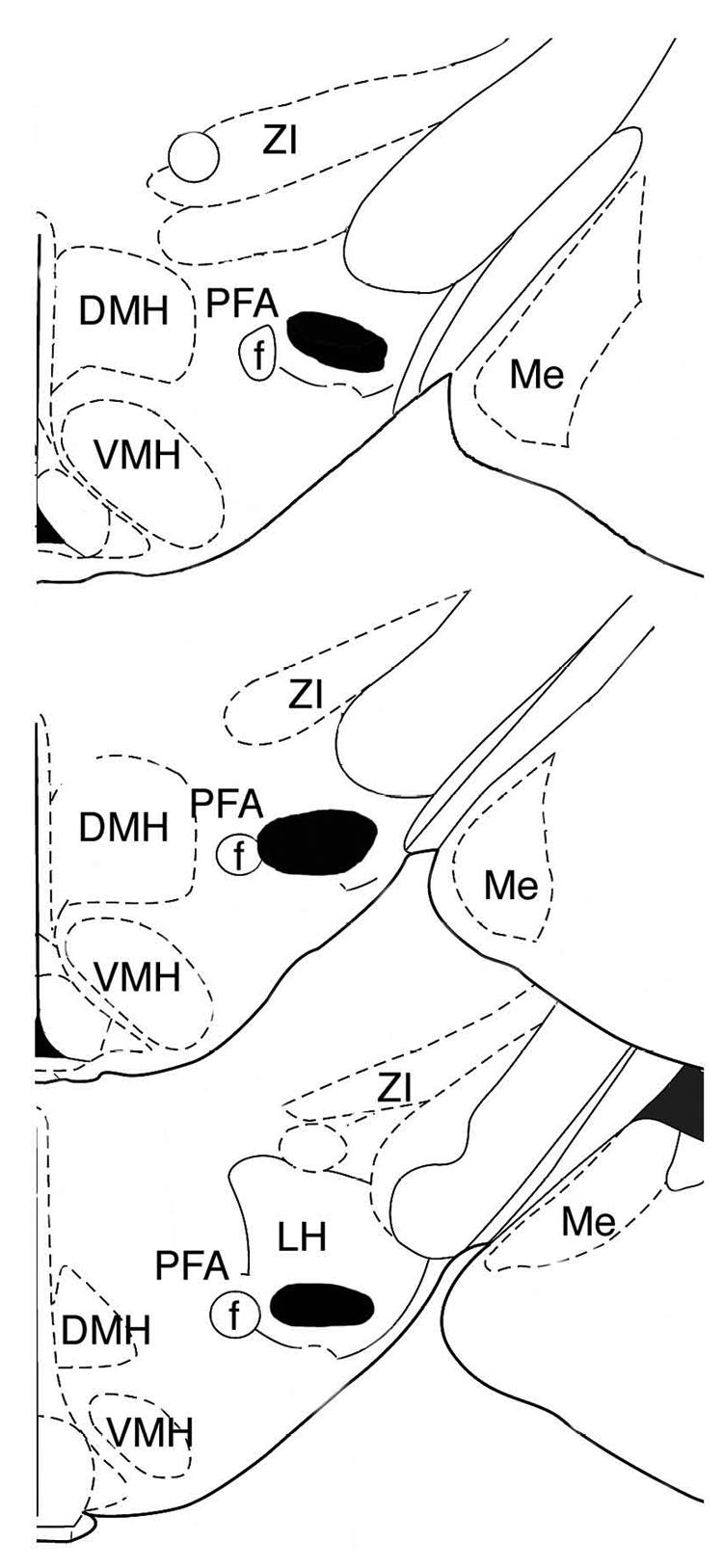
Diagram showing the extent of the effective NMDA LH orexin lesions. The black shaded area indicates the largest extent of the lesions. Drawings were adapted from Paxinos and Watson 1998. ZI=zona incerta, DMH=dorsomedial hypothalamus, LH=lateral hypothalamus, PFA perifornical area, Me= medial amygdala nuc., VMH=ventomedial hypothalamus, Arc=arcuate nucleus, f=fornix.
4. Discussion
Taken together the findings of this study indicate that LH orexin neurons play an important role in the conditioning of environmental cues to drug reward, whereas PFA orexin neurons show no association with this learning. Experiment 1 revealed that LH orexin neurons, in contrast to PFA orexin neurons, are activated by a morphine injection only when given in a novel (CPP) environmental context. Experiment 2 showed that bilateral neurotoxic lesions that eliminated more than 50% of LH orexin neurons prevented CPP learning, whereas lesions outside the area of LH orexin neurons had no effect on CPP learning. Finally, the results from Experiment 3 indicated that orexin projections specifically from the LH to the VTA regulate CPP learning.
The finding that LH orexin neurons were activated by morphine only in the presence of novel environmental cues indicates that they do not respond to drug reward per se (morphine given in the home cage) nor to a novel environmental context alone (saline given in the CPP box). Instead, we found that Fos activation above baseline only occurred with the combination of a novel environmental context and drug reward. It is worth noting that for the data collected on Days 1 and 3 animals were only given a single injection of either morphine or saline on that day. Previously, we found that LH orexin neurons were activated in proportion to preference exhibited during a CPP test for either morphine, cocaine or food reward [15]. PFA orexin neurons, on the other hand, did not show enhanced Fos activation in the presence of these same reward-associated cues, but they were preferentially activated by exposure to footshock (i.e. a highly arousing stimulus) [15] [12]. The LH orexin neurons, in contrast, were not activated by footshock [15], thus illustrating the dichotomy of function between these two populations of orexin neurons [12]. This is consistent with the finding that PFA orexin neurons become Fos activated in a graded fashion during arousing events [9], whereas LH orexin become Fos activated in a graded fashion depending on the amount of preference shown for reward-associated cues [15]. Other research has shown a relationship between Fos activation in orexin neurons locating in the LH as opposed to PFA in the induction of feeding behavior [2]. Furthermore, chronic alcohol consumption in alcohol preferring rats was found to increase the area of orexin mRNA expression the lateral but not medial hypothalamic orexin areas [21]. These findings further indicate a role for LH orexin cell groups in natural as well as drug reward.
The level of Fos activation we previously found in LH orexin neurons in the presence of reward-associated cues was similar to what we found in this study for the group given morphine in the CPP box (i.e., about 50% of orexin neurons were Fos+). In contrast, morphine given in the home cage, or saline given in the CPP context, resulted in Fos activation similar to what we [15] and others [9] reported for resting baseline (constitutive) levels in these neurons of 15 – 20%.
The present findings that Fos activation occurred during the first and third morphine conditioning trials in the CPP box, but not during saline conditioning trials nor in the home cage, together with results from our previous report [15] showing that reward-associated cues alone (the drug-free CPP test day) augmented Fos activation in LH orexin cells, indicate that activation of this system is involved in the drug/environment conditioning process. These results are also consistent with conclusions for a dichotomy in orexin function, with LH orexin neurons participating in reward processing whereas PFA orexin neurons participate in arousal and waking [12].
There was a small non-significant trend for increased Fos activation in the LH orexin neurons on day 3 of conditioning in each group. This effect could be due to a general sensitization-type response to repeated injections of morphine. However, these experiments were performed with different groups of animals and these different results may therefore simply reflect individual variability in this measure.
We also found that animals with chemical lesions of LH orexin neurons failed to show that they learned the association between drug reward and environmental context. Animals in which the majority of LH orexin neurons were lesioned bilaterally failed to exhibit a preference for the morphine-paired context, whereas animals with unilateral lesions or bilateral lesions located slightly rostral, caudal or medial (including PFA lesion) to the LH orexin neurons conditioned normally. The fact that partial lesions of the LH orexin field were effective in blocking conditioning may indicate that a subset of LH orexin neurons are involved in conditioning. In support of the idea, previously we found that only 50% of orexin neurons are activated by cues associated with drug or food reward [15]. The results of this study do not exclude the possibility that non-orexin cells located in the same vicinity as LH orexin neurons are also involved in morphine CPP conditioning. However, previously we found that Fos activation in non-orexin LH neurons did not correlate with or predict preference scores, whereas Fos activation in orexin-positive neurons showed strong correlations with, and were highly predictive of, both initial preference scores and reinstatement of preference after exogenous neuronal activation [15]. One explanation for the results found in the current experiments could be the possibility that the lesioned LH neurons are necessary to experience the rewarding effects of morphine. However, this explanation is unlikely given that the LH orexin neurons are not activated by morphine alone but are only activated in the presence of both morphine and novel environmental cues, as revealed in Experiment 1. Furthermore, non-orexin LH neurons were not consistently activated in the presence of morphine or morphine associated cues [15].
The LH is the primary origin of orexin inputs to the VTA [10], with fewer orexin afferents originating in the PFA. Furthermore, the VTA contains high levels of orexin receptors and varicosities [10] and participates in the control of behaviors related to both natural and drug reinforcers [8,36]. In this study we found that a unilateral LH orexin lesion, coupled with an infusion of the orexin antagonist SB 334867 into the contralateral VTA, completely prevented the expression of a morphine place preference. A unilateral LH orexin lesion alone (e.g., with an SB injection outside the VTA) had no effect on preference behavior. Likewise a unilateral SB injection in the VTA without a lesion of the LH orexin neurons also did not affect the learning of morphine preference and had no effects on locomotor activation. These findings indicate that bilateral projections from LH orexin neurons to the VTA are required for learning or memory of a morphine place preference. Although our lesion data establish a clear role for LH orexin neurons in morphine place conditioning, they do not rule out a role for PFA orexin neurons. However, any role of PFA orexin neurons in morphine place conditioning must be minor as lesions that spared them were unable to rescue the behavior. Conversely, lesions that spared LH orexin neurons allowed normal behavioral expression of morphine CPP. A recently published report showed that bilateral infusions of the orexin antagonist, SB 334687, into the VTA on conditioning days prevented the acquisition of a conditioned morphine place preference [25], indicating that orexin release in the VTA is necessary for learning the association between environmental cues and morphine reward. Our results are consistent with this conclusion, and in addition show that the orexin inputs originate in the LH.
A recent paper by Borgland et al. [3] provides direct evidence that orexin is involved in plasticity in VTA DA neurons and indicates a mechanism whereby orexin release in the VTA may affect drug/environment conditioning. In that study, orexin-A administration was found to acutely potentiate NMDA receptors on VTA DA neurons, which in turn led to late-phase AMPA receptor-mediated long-term potentiation (LTP) in these cells. Previously we showed that activation of either NMDA or AMPA glutamate receptors in VTA is necessary for the acquisition of conditioned place preference for cocaine or morphine [13,16]. Together, these results indicate that an orexin-glutamate interaction in the VTA may be involved in stimulus-drug conditioning. The Borgland et al. paper [3] also found that administration of an orexin-A antagonist blocked LTP that normally occurs in VTA DA neurons following cocaine administration [34], as well as the associated locomotor sensitization following repeated cocaine exposure. Together with our data, these results indicate that orexin release, originating from the LH, is associated with synaptic plasticity within the VTA and regulates drug-stimulus conditioning.
Previously we showed that stimulation of LH, but not other, orexin neurons reinstated an extinguished place preference for morphine [15]. This indicates that stimulation of these cells in the environment previously associated with morphine elicited a recall of the morphine-environment relationship, thereby producing reinstatement of preference. Moreover, in that same report we found that systemic administration of the orexin antagonist SB 334867 attenuated the expression of a place preference for morphine after it had been learned. Together, these results indicate that the LH orexin projection to the VTA play a role not only in learning stimulus-drug relationships, but also in the recall of this conditioning.
The current results support previous findings indicating a role for LH orexin neurons in reward processing, and for the first time extend this to indicate a role for this subpopulation of orexin neurons, and their projections to the VTA, in the acquisition of drug-environment relationships. This information may ultimately improve our knowledge of how environmental cues become strong motivators of behavior and drive drug-seeking behavior.
Acknowledgements
Supported by PHS grant DA017289.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- 2.Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- 3.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalcante-Lima HR, Lima HR, Costa-e-Sousa RH, Olivares EL, Cedraz-Mercez PL, Reis RO, Badaue-Passos D, Jr., De-Lucca W, Jr., de Medeiros MA, Cortes Wda S, Reis LC. Dipsogenic stimulation in ibotenic DRN-lesioned rats induces concomitant sodium appetite. Neurosci Lett. 2005;374:5–10. doi: 10.1016/j.neulet.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 7.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 9.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 11.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- 14.Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: Association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- 15.Harris GC, Wimmer M, Aston-Jones G. A novel role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 16.Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-Associated Plasticity in the Ventral Tegmental Area is Necessary for Conditioning Environmental Stimuli with Morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Katsuki H, Akaike A. Excitotoxic degeneration of hypothalamic orexin neurons in slice culture. Neurobiol Dis. 2004;15:61–69. doi: 10.1016/j.nbd.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 19.Koepp J, Lindsey CJ, Motta EM, Rae GA. Role of the paratrigeminal nucleus in nocifensive responses of rats to chemical, thermal and mechanical stimuli applied to the hind paw. Pain. 2006;122:235–244. doi: 10.1016/j.pain.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area domaminergic and nondopaminergic neurons by orexin/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 23.Majchrzak M, Ferry B, Marchand AR, Herbeaux K, Seillier A, Barbelivien A. Entorhinal cortex lesions disrupt fear conditioning to background context but spare fear conditioning to a tone in the rat. Hippocampus. 2006;16:114–124. doi: 10.1002/hipo.20138. [DOI] [PubMed] [Google Scholar]
- 24.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 25.Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman LA, Burk JA. Effects of excitotoxic thalamic intralaminar nuclei lesions on attention and working memory. Behav Brain Res. 2005;162:264–271. doi: 10.1016/j.bbr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press Inc.; San Diego, CA: 1998. [Google Scholar]
- 29.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 31.Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- 32.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050:156–162. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 33.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS letters. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 34.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 35.Winsky-Sommerer R, Boutrel B, de Lecea L. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- 36.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]


