Abstract
Background
Studies have shown that alcoholics have smaller brain volumes than non-alcoholic cohorts, but an effect of family history of heavy drinking on brain volume has not been demonstrated. We examined the relationship between a family history of heavy drinking and both brain shrinkage as measured by the ratio of brain volumes to intracranial volume (ICV) as well as maximal brain growth as measured by ICV in early-onset and late-onset alcoholics.
Methods
Using T1-weighted resonance imaging, we measured ICV, brain volume, and white and gray matter volume in adult treatment-seeking late-onset and early-onset alcoholics with either a positive or a negative family history (FH) of heavy alcohol use, and in healthy controls. We also calculated brain shrinkage using a ratio of soft tissue volumes to ICV.
Results
FH positive alcoholic patients had significantly smaller ICVs than FH negative patients, suggesting smaller premorbid brain growth. Brain shrinkage did not correlate with FH. Late-onset alcoholics showed a greater difference in ICV between FH positive and FH negative patients than early-onset alcoholics. Late-onset FH positive patients also had significantly lower IQ scores than late-onset FH negative patients, and IQ scores were correlated with ICV.
Conclusions
These data provide evidence that parental alcohol use may increase risk for alcoholism in offspring in part by a genetic and/or environmental effect that may be related to reduced brain growth.
Keywords: alcoholism, magnetic resonance imaging, neurodevelopment, brain volumes, intracranial volume, IQ, family history
Children of alcoholics (COAs) are at greater risk of developing alcoholism than children from nonalcoholic families (Cotton 1979; Devor and Cloninger 1989; Sher 1991). Many factors contribute to this increased risk. In addition to inheriting genetic predisposition, COAs may suffer from both biological and psychological injury, stemming from poor diets, inadequate psychological support, unstable parental relationships, and gestational alcohol exposure due to maternal alcohol use, all of which could contribute to the development of alcoholism (Carrion et al 2001; De Bellis et al 1999; Rosso 1990; Welch-Carre 2005). However, except in the case of fetal alcohol syndrome (FAS), direct physical evidence for the effects of the putative genetic and environmental factors mediating the family transmission of alcoholism is lacking.
Many studies have shown that alcohol-dependent men and women have smaller brain volumes than their non-alcohol-dependent cohorts (Bjork et al 2003; Jernigan et al 1991; Pfefferbaum et al 1992), but an effect of family history of heavy drinking on brain volume in alcoholism has not been demonstrated. It is widely believed that most of the difference in brain volume between alcoholics and non-alcoholics is due to ethanol neurotoxicity which causes the alcoholic’s brain to shrink with aging to a greater extent than the non-alcoholic’s. If this is true then a family history of heavy drinking could only contribute to differences in brain volume between alcoholics and non-alcoholics by altering an individual’s vulnerability to ethanol neurotoxicity or by causing alcoholics with a family history of heavy drinking to drink more than alcoholics without a family history. However, it is not clear that the difference in brain volume between alcoholics and non-alcoholics is due exclusively to ethanol neurotoxicity.
We and others have reported that alcoholics have smaller intra-cranial volumes (ICVs) than non-alcoholics (Bjork et al 2003; Cardenas et al 2005; Hommer 2003). These differences are around 2.5 % but they do not reach statistical significance. The small difference in ICV we observed between alcoholics and non-alcoholics suggested that there could be a subgroup of alcoholics, such as COAs, with considerably smaller ICV. Unlike brain volume itself, ICV is a valid measure of brain growth because it is determined by skull growth, which occurs as the brain, meninges, and cerebrospinal fluid space expands to their maximal size around puberty (Carmichael 1990). ICV does not change as a function of neurodegeneration or aging like brain volume (Jenkins et al 2000), and therefore is a useful estimate of the lifetime maximum volume of the brain (Blatter et al 1995). Though ICV is highly heritable, it may also be influenced by environmental conditions (Baare et al 2001), particularly when the environment is not ideal. There have been several animal studies demonstrating gestational exposure to ethanol causes decreased size of craniofacial structures (Edwards and Dow-Edwards 1991), and a small head size is one of the diagnostic criteria for FAS (Mattson et al 1996; Roebuck et al 1998). Some COAs who are not formally diagnosed with FAS may have fetal alcohol effects which are more subtle and may include slight reductions in skull and brain size
In this study, we used T1-weighted magnetic resonance imaging to measure ICV, cerebral volume, white and gray matter volume in both healthy controls and in adult treatment-seeking alcoholics with and without a positive family history (FH) of heavy drinking. We also further analyzed ICV, as well as soft tissue volumes, within the FH positive alcoholics as a function of which parent was a heavy drinker (neither, mother, father, or both) in order to determine if the alcohol use of each parent had a differential influence on brain growth and development. A study in rats by (Abel 1993) demonstrated that even in alcohol-treated males who sired offspring, there was a significant increase in the number of “runts,” or smaller than average offspring, at birth compared to those sired by non-alcohol-treated males. We therefore hypothesize that a positive FH, even one limited to fathers alone, would be associated with smaller ICV, but those alcoholics with a maternal FH of heavy drinking would be most severely affected.
In addition to premorbid differences in brain growth as indexed by ICV, we also examined whether a family history of heavy alcohol use was independently related to the amount of brain shrinkage which occurs throughout adulthood. A previous study by Cardenas et al (2005) found a positive FH to be protective against brain shrinkage in heavy drinkers. In our study, brain shrinkage was be inferred from the ratio of cerebral volume to total ICV. If FH does affect ICV, then the smaller absolute brain volumes observed in alcoholics may be a result of either greater brain shrinkage with age, smaller maximal brain growth or both. Calculating a ratio of brain volume to ICV allows us to independently measure the contribution FH makes to brain shrinkage as well as brain growth.
Finally, many studies have shown a weak but consistent correlation between brain size and IQ (e.g. Andreasen et al 1993; De Bellis et al 1999; Willerman 1991) and several studies indicate that COAs tend to do more poorly academically than control children (Ervin et al 1984), particularly in verbal skill (Gabrielli and Mednick 1983). These cognitive/reasoning deficits may be related to a reduction in brain size in COAs. If a positive FH is correlated with decreased brain sizes and general neurodevelopmental deficits, we would observe lower IQ scores in FH positive alcoholics. We conducted IQ tests in order to see if ICV and a positive FH are accurate predictors of intelligence.
Methods
Participants
Alcohol-dependent patients were recruited from among all the patients consecutively admitted to the National Institute of Alcohol Abuse and Alcoholism (NIAAA) inpatient unit at the Clinical Center of the National Institutes of Health in Bethesda, MD between January 1995 and September 2004. Most patients lived in Montgomery County, MD, or the greater Washington, DC area. All participants were interviewed using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders. Information on recent and chronic alcohol use was obtained from structured research questionnaires. All subjects provided written informed consent to participate in the study which was approved by the NIAAA Institutional Review Board.
All alcoholic patients met DSM-III-R criteria for alcohol dependence. We excluded patients who met the criteria for alcohol abuse but not alcohol dependence, as well as those who had a history of delirium tremens or gross neurological disorders. In addition, we excluded patients who had an IQ less than 80 or who demonstrated signs of dementia or Korsakoff’s disease. Participants were not thiamine deficient at admission, and none of the subjects had a history of head injury requiring hospitalization. Patients were scanned three weeks after admission, or if they had been transferred from another hospital, at least three weeks from the last alcohol use.
Family history was assessed by administering an interviewer-completed Lifetime Drinking questionnaire after the patient had undergone several weeks of group therapy focused on alcoholism. The family history of the participant was determined through the use of a rating instrument with six categories ranging from “does not drink” to “alcoholic.” If a patient rated his or her biological father or mother as: a “heavy drinker,” “problem drinker,” or “alcoholic,” the patient was considered to have a positive FH. We further subdivided patients according to age of onset of alcohol-dependence. Age of onset of alcoholism was defined as the age at which the patient first consumed 90 drinks in a one-month period. Early onset alcohol-dependent patients (EOAs) had an age of onset of alcoholism between the ages of 13–25 years, and late onset alcohol-dependent (LOAs) had an age of onset of alcoholism greater than 25 years of age. Average quantity (average number of drinks daily per drinking day) and frequency (number of drinking days per month) were also calculated over the six month period preceding admission. Years of heavy drinking was defined as the cumulative total contiguous or noncontiguous months during which the subject drank 90 drinks per month (note: since subjects often maintain this high a level of alcohol use for at least 12 consecutive months, months were summed into years). Patients were considered comorbid substance abusers if they met DSM-III or DSM-IV criteria for drug abuse or dependence with a substance other than alcohol at some point in their life.
Healthy community-recruited male and female participants with no history of significant medical illness or psychiatric disorders were included for comparison. Most control participants were also drawn from the Montgomery County, MD and greater Washington DC area. All participants were assessed with the Structured Clinical Interview for either DSM- III-R or DSM-IV, which confirmed that each patient met criteria for alcohol dependence and that no comparison subject met criteria for a psychiatric disorder.
Magnetic resonance imaging scan acquisition and analysis
Participants were scanned with 1.5 T MRI (GE Medical Systems, Milwaukee, Wisconsin) using a fast spoiled-GRASS (FSPGR) sequence. A gapless series of high contrast 2 mm thick T1-weighted coronal images (repetition time, 25 msec, inversion time, 5 msec and echo time, 16 msec) was obtained. Images were acquired using a 256 by 256 matrix with a 240 by 240-mm field of view. Each volumetric brain consisted of 124 coronal slices with voxel size of 0.9375 by 0.9375 by 2.0 mm.
Intracranial tissue margins were marked manually on coronal sections with a hand-driven cursor. The ICV included the cerebrum and cerebrospinal fluid (CSF) spaces covering the cortex, but excluded the cerebellum and CSF of the posterior fossa. Inter-rater reliability for manual identification of the ICV of 10 randomly selected MRI volumes was high (intra-class correlation = .97). Next, brain tissue was automatically segmented into gray matter, white matter, sulcal CSF, and ventricular CSF using a previously described computerized method (Momenan 1997) that used voxel intensity to perform a K-means clustering procedure. Cerebral brain volume was calculated by adding the white and gray matter volumes. Brain shrinkage was inferred by calculating the ratio of cerebral volume, gray volume, and white volume to total ICV.
Intelligence (IQ)
IQ was estimated using the WAIS-R vocabulary and block design tests (Wechsler 1981). IQ data was available for 203 alcoholic participants. The vocabulary test measured verbal intelligence, and the block design tested visuospatial abilities by requiring the subject to create geometric designs using blocks. These two subtests have previously been used as a “short-form” of the WAIS-R to estimate IQ (Silverstein 1983) and results of the short form significantly correlate with scores of the Full Scale test (Silverstein 1985). Age-corrected scaled scores were used to calculate estimated IQs.
Statistical Analyses
Data distributions were examined for normality. We used a general linear model (GLM) to examine the independent variables of sex, height, age, family history, age of onset of alcoholism, and all possible interactions on the dependent variables of ICV, brain volumes, and brain shrinkage as measured by the brain volume to ICV ratio (package JMP-SAS, SAS Institute; Cary, NC). We also used a GLM to test the independent variables of ICV, level of education, age, family history, and age of onset, as well as all interactions, on IQ scores. When an interaction was observed, we conducted post-hoc simple-effects analyses using a Students t-test. All significance testing was two-tailed with alpha = 0.05. In our first analysis, we divided patients into two groups, FH positive and FH negative, according to responses on the lifetime drinking history interview, and within those two groups, into late and early-onset alcoholics (LOAs and EOAs). When we found a significant FH effect, we conducted a secondary analysis where we divided patients into four groups depending on which parent was the heavy drinker (neither, mother, father, or both). In this secondary analysis, because of smaller sample sizes, we did not divide patients into LOAs and EOAs.
Results
Participant characteristics are described in table 1. We did not find any main effects of age of onset or FH on the quantity or frequency of drinking during the six months preceding hospitalization (table 2). There were no differences in the quantity or frequency of drinking between males and females when we controlled for body size, but females had a later average age of onset than males (F = 4.69, p = 0.03).
Table 1.
Demographic Characteristics of Study Groups
| Variable | Late Onset alcoholics (n = 102) | Early Onset alcoholics (n = 129) | Controls (n = 114) | |||
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean (SD) | 43.90 (8.48) | 36.98 (8.65) | 34.63 (10.13) | |||
| Range | 28–67 | 20–64 | 20–63 | |||
| Sex | ||||||
| Male | 51 | 104 | 54 | |||
| Female | 51 | 25 | 60 | |||
| Education | ||||||
| Mean Years | 14.92 (2.62) | 13.79 (2.55) | 16.92 (2.72) | |||
| Height (cm) | 169.34 (9.26) | 172.51 (8.52) | 168.08 (13.64) | |||
| Ethnicity | ||||||
| Caucasian | 83 | 105 | 83 | |||
| Black | 17 | 18 | 16 | |||
| Hispanic | 1 | 3 | 7 | |||
| Asian | 1 | 0 | 6 | |||
| Other | 0 | 3 | 2 | |||
| Family History | ||||||
| FH − | 38 | 47 | 114 | |||
| FH + | 64 | 82 | 0 | |||
| Mother | 12 | 12 | ||||
| Father | 35 | 42 | ||||
| Both | 17 | 28 | ||||
Table 2.
Drinking Behavior and Co-morbid Drug Abuse of Study Groups
| Early-Onset | Late-Onset | |||
|---|---|---|---|---|
| FH − | FH + | FH − | FH + | |
| Mean Age of Onset (SD) | 18.98 (2.49) | 19.32 (2.87 | 33.76 (7.45) | 33.03 (7.47) |
| Range | 14–24 | 13–24 | 25–55 | 25–59 |
| Mean Quantity Consumed | 14.87 (6.87) | 13.46 (7.3) | 11.23 (5.87) | 11.69 (7.07) |
| Mean Drinking Frequency | 26.08 (6.94) | 21.9 (10.98) | 24.34 (8.85) | 25.37 (8.13) |
| Mean Years of Heavy Drinking | 15.07 (7.94) | 13.04 (7.19) | 8.65 (6.59) | 8.79 (6.81) |
| % Comorbid Drug Abusers | 72 % | 71 % | 39 % | 48 % |
Psychiatric history is summarized in table 3. Early-onset alcoholics had a greater number of total Axis II disorders than late onset alcoholics (F = 18.05, p < 0.001), but there were no differences in total number of mood or anxiety disorders. There was no effect of FH on psychiatric diagnoses, or on the percentage of comorbid drug abusers. Early-onset alcoholics were significantly more likely than late-onset alcoholics to have abused drugs other than alcohol (F = 29.08, p < 0.0001).
Table 3.
Psychiatric Diagnoses of Study Groups
| Total # Lifetime Disorders | Early-Onset | Late-Onset | |||
|---|---|---|---|---|---|
| Mood | FH − | FH + | FH − | FH + | |
| 0 | 13% | 27% | 14% | 14% | |
| 1 | 53% | 31% | 32% | 46% | |
| 2 | 28% | 35% | 40% | 33% | |
| 3 | 4% | 6% | 13% | 6% | |
| Mean (SD) | 1.30 (2.58) | 1.27 (3.02) | 1.54 (2.89) | 1.49 (1.92) | |
| Anxiety | |||||
| 0 | 55% | 53% | 67% | 55% | |
| 1 | 30% | 32% | 19% | 35% | |
| 2 | 13% | 12% | 8% | 7% | |
| 3 | 2% | 2% | 5% | 2% | |
| Mean (SD) | 0.62 (0.83) | 0.64 (1.12) | 0.51 (0.90) | 0.62 (1.17) | |
| Axis II | |||||
| 0 | 10.5% | 14.8% | 40% | 21.8% | |
| 1 | 19.1% | 13.6% | 10.8% | 21.8% | |
| 2 | 8.5% | 11.1% | 8% | 18.2% | |
| 3 | 12.8% | 9.9% | 16.2% | 9.1% | |
| 4 | 10.6% | 7.4% | 5.4% | 10.9% | |
| > 4 | 38.5% | 43.2% | 19.6% | 18.2% | |
| Mean (SD) | 3.62 (0.80) | 4.0 (0.80) | 2.41 (0.87) | 2.25 (0.85) | |
Intracranial volume
We found a significant difference in ICV among healthy controls, FH positive, and FH negative alcohol-dependent patients (F (2, 356) = 6.52, p = 0.0017). Post-hoc student’s t-tests demonstrated a significant difference between controls and FH positive alcoholics (p < 0.001), but not between controls and FH negative alcoholics (figure 1). A Least Squares Fit model showed that sex, height, and FH, as well as the interaction between FH and age of onset, independently accounted for significant proportions of the variance in ICV (table 4). We found a significant difference in ICV as a function of family history in both male and female alcoholics (figure 2). We did not find significant differences between the ICVs of EOAs and LOAs. There was a significant interaction effect of FH X age of onset (F (2,356) = 5.209. p = 0.023). In a post-hoc simple effect tests, the ICVs of FH negative LOAs were significantly greater than the ICVs of FH positive LOAs (p < 0.0001). We did not find a significant difference in the ICV of FH positive compared to FH negative EOAs. Furthermore, we did not find a significant difference in ICV between EOAs and LOAs with a positive FH, but within FH negative subjects, LOAs had significantly larger ICVs than EOAs (p = 0.02) (figure 3).
Figure 1.
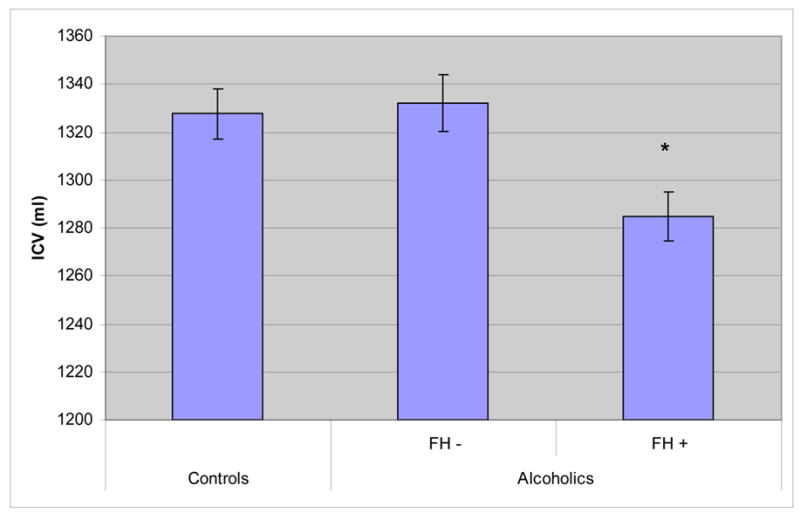
Adjusted Means of ICV in Controls and Alcoholic Patients. We found a significant difference in ICV among healthy controls, FH positive, and FH negative alcoholic patients (F = 6.52, p = 0.0017). Post-hoc student’s t-tests demonstrated a significant difference between controls and FH positive alcoholic patients.
Table 4.
Factors Affecting Brain Volume Measures in Alcoholic Patients
| r2 | Sex | Height | Age | Family History | Age of Onset | Family History X Age of Onset | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| complete model | F | p | F | p | F | p | F | p | F | p | F | p | |
| ICV | 0.33 | 20.277 | < 0.0001 | 11.884 | 0.001 | 0.01 | 0.92 | 13.232 | < 0.0001 | 0.379 | 0.539 | 5.209 | 0.023 |
| Brain/ICV | 0.30 | 5.692 | 0.0179 | 0.0176 | 0.8945 | 61.337 | < 0.001 | 2.119 | 0.1469 | 0.001 | 0.979 | 0.765 | 0.383 |
| Gray/ICV | 0.39 | 1.557 | 0.213 | 2.639 | 0.105 | 105.748 | < 0.001 | 1.674 | 0.197 | 0.1194 | 0.729 | 1.716 | 0.191 |
| White/ICV | 0.19 | 9.917 | 0.002 | 2.245 | 0.135 | 13.427 | < 0.001 | 1.81 | 0.179 | 0.106 | 0.744 | 0.209 | 0.647 |
The value of r2 equals the amount of variance explained by all of the factors (sex, height, age, family history, and age of onset) included in the model.
Figure 2.
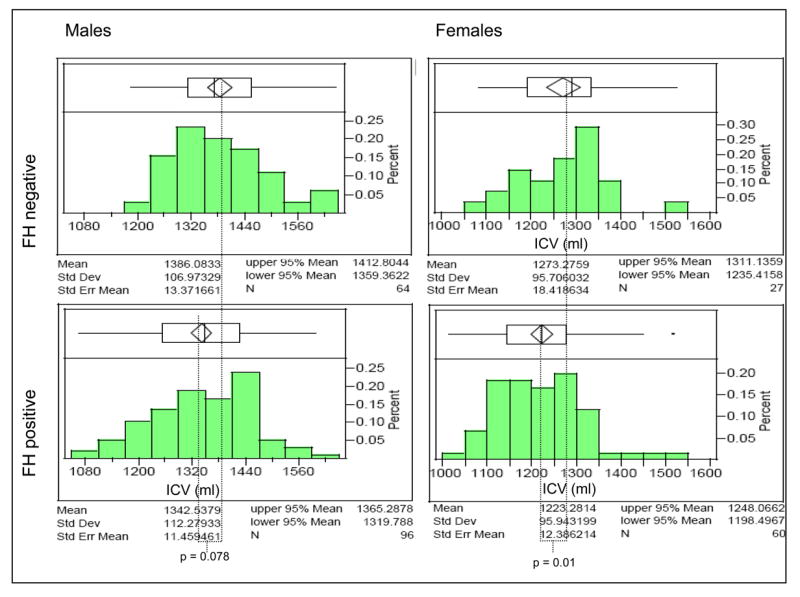
ICVs of Male and Female Alcoholic Patients. The rectangular box above each distribution shows the middle half of the data. The solid line within the box represents the median value. The whiskers that extend out from the box show the tails of the distribution, and any points outside of the whiskers are possible outliers. The solid line connecting the FH+ and FH− panels represents the mean value for each cell.
Figure 3.
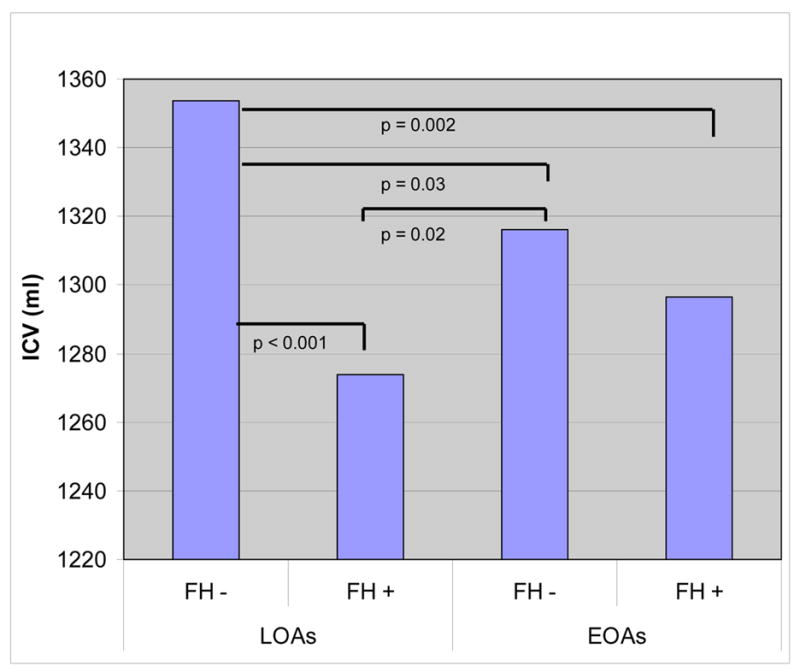
Adjusted means of intracranial volume in LOAs and EOAs. We found a significant effect of FH (F = 13.23, p < 0.0001) and an interaction between FH and age of onset (F = 5.209, p = 0.023). Bars indicate significant results of a student’s t-test among the four groups.
In the second analysis, we divided alcoholic patients into four groups depending on which parent was a heavy drinker, again controlling for height, sex, and age. This model demonstrated a significant difference in ICV among the four groups (F (3, 242) = 4.521, p = 0.004). Pairwise post-hoc t-tests found that alcoholics with no FH had significantly larger ICVs than those with a heavy drinking father (p = 0.0133), a heavy drinking mother (p = 0.0104), and two heavy drinking parents (p = 0.0037). Moreover, FH negative males had larger ICVs than males with a heavy drinking mother or father (p < 0.05). In contrast, among females, FH negative patients had larger ICVs than those with a heavy drinking mother or both heavy drinking parents (p < 0.05), but there was no difference between female patients with a heavy drinking father and those who were FH negative (figure 4).
Figure 4.
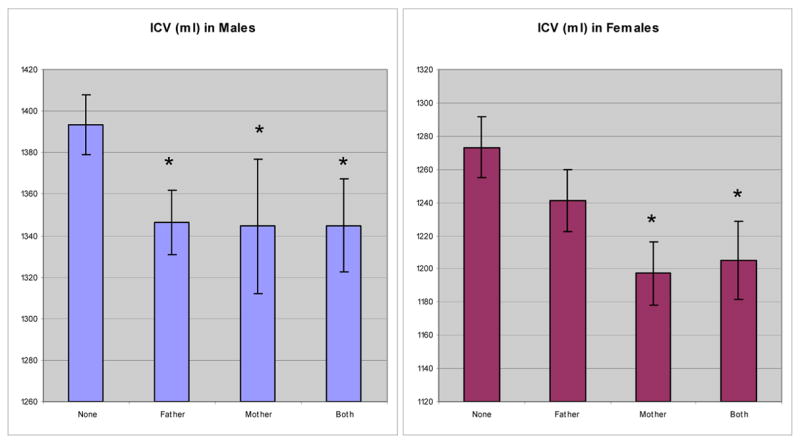
Adjusted means of ICV in alcoholic patients. We generated adjusted means for ICV after co-varying for sex and height. The ICVs of FH negative patients were significantly larger than those of FH positive patients. An asterisk indicates that the mean is significantly different from “none.”
Brain Shrinkage
We found no main effects of FH or of age of onset of alcoholism on brain shrinkage (the brain volume/ICV ratio), and no interaction between the two measures (table 5). Predictors of brain shrinkage included age (F = 57.67, p < 0.0001), sex (F = 15.11, p = 0.0001), and years of heavy drinking (F = 5.02, p = 0.02). Age of onset of alcoholism did not significantly correlate with brain shrinkage in either males or females. Female alcoholics experienced significantly lower ratios of brain volume to ICV, indicating greater shrinkage, than males. There were no significant interactions between sex and either family history or age of onset. When we examined selective shrinkage of gray and white matter volumes we found similar results, but both FH positive and FH negative alcoholics had greater brain shrinkage than healthy controls (F (1, 356) = 69.75, p < 0.0001).
Table 5.
ICV and Brain Shrinkage Values in Healthy Controls and Alcoholic Patients
| Controls | FH − | FH + | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| ICV | 1323.89 | 129.65 | 1349.89 | 113.14 | 1296.48* | 121.28 |
| Brain/ICV | 0.824* | 0.028 | 0.790 | 0.032 | 0.796 | 0.034 |
| Gray/ICV | 0.441* | 0.021 | 0.419 | 0.020 | 0.423 | 0.021 |
| White/ICV | 0.382* | 0.035 | 0.371 | 0.016 | 0.372 | 0.017 |
An asterisk indicates a significant difference (p < 0.05) from the other two groups.
IQ
Total IQ scores were predicted significantly and independently by ICV, level of education, and by FH, but not by sex, age, or age of onset (see table 6). When examined separately block design and vocabulary scores both were predicted by age. However, vocabulary significantly increased with age while block design score decreased. In addition to age, vocabulary score was also predicted by ICV, education and FH. In contrast, block design score was not predicted by ICV but was predicted by education and FH.
Table 6.
Factors Affecting Estimated IQ in Alcoholic Patients
| r2 | ICV | Education | Age | Family History | Age of Onset | Family History x Age of Onset | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| complete model | F | p | F | p | F | p | F | p | F | p | F | p | |
| Total Estimated IQ | 0.27 | 4.389 | 0.038 | 22.587 | < 0.0001 | 2.087 | 0.151 | 7.62 | 0.007 | 0.163 | 0.163 | 4.85 | 0.029 |
| Vocabulary IQ | 0.35 | 4.025 | 0.042 | 27.158 | < 0.0001 | 4.271 | 0.041 | 5.857 | 0.017 | 0.070 | 0.792 | 1.596 | 0.208 |
| Block IQ | 0.19 | 0.398 | 0.529 | 6.344 | 0.013 | 14.839 | 0.0002 | 3.993 | 0.049 | 0.854 | 0.357 | 5.646 | 0.019 |
The value of r2 equals the amount of variance explained by all of the factors (ICV, education, age, family history, and age of onset) included in the model.
There was a significant interaction between the age of onset and parental drinking in both performance (block design) IQ and total IQ. For total IQ, posthoc student’s t-tests indicated that FH positive LOAs had significantly lower scores than FH negative LOAs, but there was no significant difference between any of these measures in EOAs as a function of family history (figure 5). In addition, FH negative LOAs scored significantly higher than FH positive EOAs. In block design score, the same pattern emerged, with FH positive LOAs scoring significantly lower than FH negative LOAs, but no difference in the scores of EOAs as a function of FH.
Figure 5.
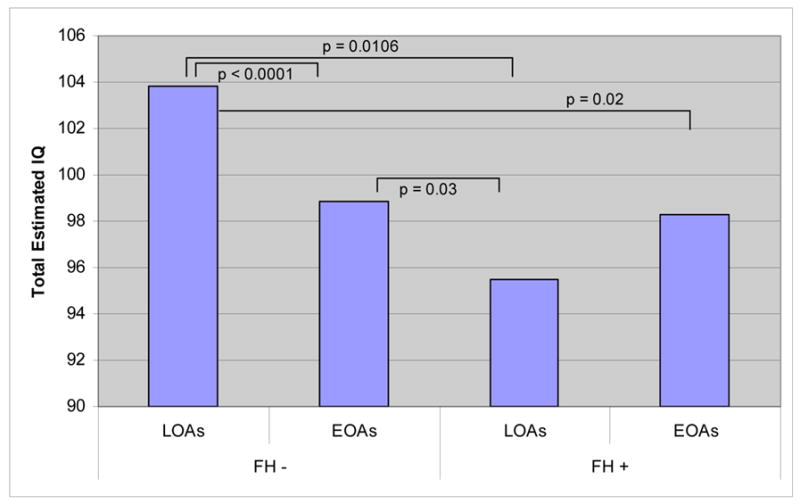
Estimated IQ Scores of EOAs and LOAs by Family History. We found a significant effect of FH (F = 11.202, p = 0.001) and an interaction between FH and age of onset (F = 8.85, p = 0.003). Bars indicate significant results of a student’s t-test among the four groups.
Analyses conducted with patients divided into four groups again demonstrated that total IQ scores differed significantly as a function of FH (F (3,203) = 5.11, p = 0.002) (figure 6). Post-hoc student’s t-tests indicated that FH negative patients had significantly higher scores than the FH positive patients. FH negative patients had higher block design scores, but the difference did not reach significance. In vocabulary scores, there was a significant difference as a function of FH (F (3, 203) = 4.48, p = 0.005), and student’s t-tests indicated that FH negative patients had higher scores than FH positive patients (p = 0.014).
Figure 6.
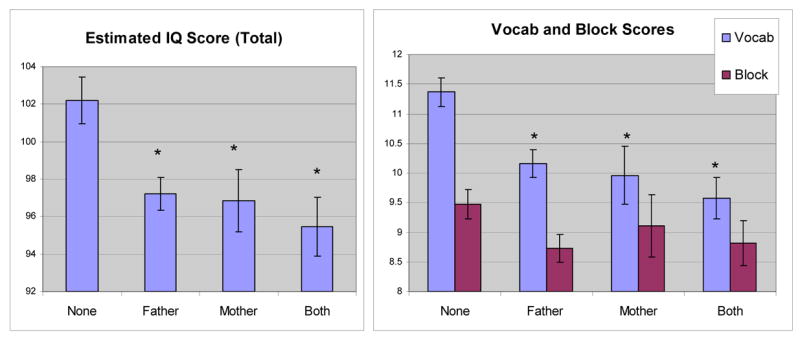
Estimated IQ Scores of Alcoholic Patients. (Left) FH negative patients had significantly higher scores than FH positive patients. (Right) FH negative patients had significantly higher vocabulary scores than FH positive patients. An asterisk indicates that the mean is significantly different from “none.”
Discussion
The main finding of this paper is that adult alcoholics with a positive FH of heavy drinking have significantly smaller ICVs than alcoholics from non-alcoholic or heavy drinking families when we controlled for age, sex, and height. Brain shrinkage as measured by the ratio of brain volumes to ICV was not affected by FH. Only maximal brain and skull growth as measured by ICV was affected by FH. FH did not correlate with drinking behavior of the alcoholics themselves. Although drinking patterns may have varied throughout the lifetimes of the patients, there were no significant differences in the frequency of drinking, the quantity of drinking, total years of heavy drinking, or the age of onset of heavy drinking between the patients with a positive FH and those without. This suggests that differences in ICV between FH positive and negative alcoholics are not the result of different drinking patterns. Also, since the mean age of onset of heavy drinking, even for the EOAs, was more than 2 SDs greater than the age at which ICV growth typically ends, it is unlikely that heavy drinking contributed to differences in ICV. Less skull growth may have functional consequences in that there is a correlation between IQ and brain size (Andreasen et al 1993; De Bellis et al 1999). We found that FH positive patients had significantly lower IQ scores than patients with no parental drinking and that ICV weakly, but significantly, predicted both total IQ and vocabulary score.
The relationship between ICV and intelligence should be interpreted cautiously. Although ICV is highly heritable with an h2 (the proportion of phenotypic variation that can be attributed to genetic causes) of about 0.9 (Baare et al 2001) ICV may be influenced by environmental factors as well. In fact, recent studies of the heritability of IQ have found that h2 is highest when environment is optimal but is considerably lower when estimated in populations enjoying less than ideal environments (Turkheimer et al 2003). Since ICV predicts IQ it may show a similar pattern. In addition, it seems likely that alcoholics, in general, are raised in less than optimal environments. Thus, an h2 of 0.9 for ICV may be an overestimate in alcoholic populations. However, the mechanisms by which environment affects ICV are uncertain.
Many studies have found that living in an enriched environment positively influences central nervous system growth and development (van Praag et al 2000), while other studies have described the effects of stress on brain growth, which indicate that increased cortisol and catecholamine concentrations can modulate neuronal migration, differentiation, and synaptic proliferation in the developing brain (Lauder 1988; Sapolsky 1990; Sapolsky et al 1986; Todd 1992). In both human (Sapolsky 1996; Sapolsky et al 1986), and non-human primates (Uno et al 1989) elevated levels of stress hormones such as catecholamines and cortisol can affect brain growth by accelerating loss of neurons (Swaab et al 2005) or by delaying myelination (Dunlop 1997). COAs may experience this stress during a particularly crucial developmental stage. Between the ages of 6 months and 3 years, myelination increases dramatically and continues to increase into the third decade of life, and grey matter and limbic structures increase in volume throughout this time (Sowell et al 1999). Therefore, the stress of growing up in an alcoholic home may affect brain growth and development, and correlate with increased risk for alcoholism during adulthood. DeBellis et al (1999) found that in maltreated children with post-traumatic stress disorder, cortisol and catecholamine concentrations correlated with the duration of maltreatment. In a subsequent study, they also found that decreased ICV was associated with the duration of maltreatment, and they propose that traumatic childhood experiences may adversely influence brain development. This is consistent with the measured heritability of ICV being lower in a more adverse environment. Although in the current study, it is not known whether children of heavy drinkers have experienced abuse or neglect, it is likely that they grew up in a more stressful environment than children of non-drinking parents. Most likely genetics and environment both contribute to the smaller ICV observed in FH positive alcoholics.
A surprising finding in the study was that the brain volumes of LOAs showed a greater effect of parental alcohol use than those of EOAs. This is, in large part, due to the FH negative EOAs having significantly smaller ICVs than the FH negative LOAs. This difference in ICV among FH negative alcoholic groups may be related greater severity of alcoholism among the EOAs. In a clinical setting, EOAs often have more psychopathology and poorer global functioning regardless of whether their parent is a heavy drinker (von Knorring et al 1987). The EOAs in our sample had significantly higher rates of comorbid drug abuse and dependence than the LOAs as well as a considerably higher incidence of Axis II personality disorders. In a previous study with a subset of patients of the current study, EOAs scored higher on measures of impulsivity and aggression (Bjork et al 2004). This more pathological, higher severity group may not manifest the effects of family history as clearly as other factors underpinning severe psychiatric comorbidity. Consistent with this explanation we did not find significant differences in ICV between FH positive and negative EOAs.
LOAs, in contrast, tend to have higher scores in global functioning, and alcoholism often manifests in the absence of other disorders. They have few if any social complications, few legal difficulties, and rarely act out violently while intoxicated (von Knorring et al 1987). Perhaps the differences between LOAs with and without parental heavy drinking are magnified because of the lack of other confounding factors in this “cleaner” population. Further studies are required to more thoroughly understand this effect. However, our results challenge the assumption that the genetic contribution to alcoholism necessarily manifests early in life. These data indicate that both genetics and early life environment may have profound implications that may not surface until adulthood.
We also found that among women, maternal drinking appeared to influence ICV more than paternal drinking. This makes sense, as the mother was probably the principal caretaker of the child, and more likely influence the child’s nutrition, social surroundings, and intellectual environment than the father. In addition, we have no way of assessing whether the heavy drinking mothers drank while pregnant. Although none of our participants were diagnosed with FAS, patients may have had subtle fetal alcohol effects. We did not find differences between the effects of maternal and paternal drinking on ICV in the males in our study, suggesting that at least among males fetal alcohol effects cannot explain the smaller ICV among FH positive alcoholics. We also report larger effect size for FH on ICV among the women in our sample compared to the men, perhaps due to the more selective effect of maternal drinking on females. This suggests that women may be particularly vulnerable to either prenatal alcohol effects or postnatal environmental effects.
We found no difference in brain shrinkage between EOAs and LOAs when we controlled for age, sex, and years of heavy drinking. Brain shrinkage is independently correlated with the duration of heavy drinking after controlling for age (Bjork et al 2003), but it appears that the time at which the drinking is initiated does not affect this process. The brains of LOAs appear to be just as susceptible to atrophy as those of younger alcoholics, which provides additional evidence that ICV reflects pre-morbid brain growth that is not sensitive to individual differences in the patient’s drinking behavior. Even within the alcoholics who began heavy drinking before the age of 21 (n = 85), age of onset of alcoholism did not significantly predict brain shrinkage in either males or females. We also found that as in previous work (Hommer 2001), females are more susceptible to alcohol-induced brain shrinkage at similar alcoholism severity.
We did not find a main affect of FH on brain shrinkage, indicating that brain shrinkage occurs as a result of heavy drinking regardless of FH status. This finding contrasts with a previous study by Cardenas et al (2005), which reported that a positive FH of alcoholism was protective against brain shrinkage. However, although the Cardenas paper also looked at the effects of FH of alcoholism on brain atrophy, their methods and study population differed considerably from ours. Their primary measure of brain shrinkage was % CSF, whereas ours was a ratio of brain volume to ICV. They measured the four lobes of the brain, whereas we measured total gray matter and total white matter. In addition, their population was non-treatment seeking and they drank considerably less than our population. Therefore, their findings that family history may be protective may only be valid to a certain alcoholism severity.
Estimated IQ
Consistent with Gabrielli and Mednick (1983), we found a significant effect of FH on estimated IQ after controlling for sex, age, and education level. Gabrielli and Mednick demonstrated that children at high risk for alcoholism had lowered verbal ability, suggesting that the lower IQs observed in alcoholics may exist before the onset of alcoholism. Since ICV is set before the onset of alcoholism this is consistent with our finding that that a lower vocabulary score is associated with smaller ICV (although, it should be noted, ICV is a fairly weak predictor of IQ when education, FH and age of onset are taken into account). Block score results were not as strongly correlated with either education or FH as vocabulary results. Several studies have examined the effect of parental neglect on IQ. Cognitive, language, and intellectual impairments are frequently observed in abused and neglected children (Augoustinos 1987; Kolko 1992), and the effects may reach adulthood. In a study of adult survivors of child abuse, Perez and Widom (1994) reported lower IQ and decreased reading ability in the abused group compared to controls.
Interestingly, we found that a positive FH affected the IQ scores of the LOAs, but not of the EOAs. Again, this may be explained by the greater psychopathology of the EOAs mitigating the effect of FH on IQ through a ceiling effect. FH negative LOAs had significantly higher IQ scores than FH negative EOAs, but in the FH positive patients, both EOAs and LOAs had similar low IQ scores.
Finally, we found that EOAs had significantly higher numbers of axis II disorders than LOAs, which has been shown in many clinical samples of alcoholics (Hallman et al 1996; von Knorring et al 1987). There was no main effect of FH, suggesting that parental heavy drinking does not influence the psychiatric diagnoses of adult alcoholics.
A limitation of this study was the reliance on patients’ reports of parental heavy drinking as well as use of an in-house interview instrument which did allow for a formal diagnosis of alcohol abuse or dependence. However, by the time of the interview, patients had undergone weeks of educational alcoholism therapy sessions which directly and indirectly clarify what constitutes problematic levels of drinking. On the other hand, our classification of FH positive patients as having a “heavy drinking” parent underscores the strength of the relationship between parental drinking and ICV. Even if the heavy drinking parents would not have been diagnosed with alcohol dependence, patients raised by parents with a general pattern of heavy drinking are still affected.
An additional limitation of this study is the absence of data collected about aspects of parental lifestyles other than drinking that may have contributed to smaller ICVs of offspring, such as comorbid drug abuse or socio-economic status. We were also unable to assess maternal drinking during pregnancy. We also cannot say how well FH, ICV and IQ will predict the development of alcoholism. These are risk factors, but as with any risk factor, they do not determine that a person will develop the condition, but rather increase the likelihood that they will. To answer the question of how selective risk factors predict alcoholism we would need to select FH positive subjects on the basis of low IQ or small ICV and see if they have a higher rate of alcoholism.
Future research could more precisely study how the amount of parental drinking affects brain volumes of COAs before they are old enough to develop alcoholism themselves. Subsequent studies could also examine brain volumes of healthy controls with alcohol-dependent parents, in order to determine if smaller ICV is a more specific risk factor for the development of alcoholism than FH. Finally, more in-depth psychosocial interviewing could more directly assess parental factors on both structural development and behavioral consequences in COAs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Rat offspring sired by males treated with alcohol. Alcohol. 1993;10:237–242. doi: 10.1016/0741-8329(93)90042-m. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze V, 2nd, O’Leary DS, Alliger R, Cohen G, et al. Intelligence and brain structure in normal individuals. Am J Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Augoustinos M. Developmental effects of child abuse: recent findings. Child Abuse Negl. 1987;11:15–27. doi: 10.1016/0145-2134(87)90029-9. [DOI] [PubMed] [Google Scholar]
- Baare WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJ, Schnack HG, et al. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Grant SJ, Hommer DW. Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: effects of drinking history and comorbid substance use disorder. Am J Psychiatry. 2003;160:2038–2045. doi: 10.1176/appi.ajp.160.11.2038. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Carmichael A, editor. Physical development and biological influences. Elsevier; Amsterdam: 1990. [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: a review. J Stud Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Devor EJ, Cloninger CR. Genetics of alcoholism. Annu Rev Genet. 1989;23:19–36. doi: 10.1146/annurev.ge.23.120189.000315. [DOI] [PubMed] [Google Scholar]
- Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. Journal of Maternal-Fetal and Neonatal Medicine. 1997;6:309–313. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<309::AID-MFM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Edwards HG, Dow-Edwards DL. Craniofacial alterations in adult rats prenatally exposed to ethanol. Teratology. 1991;44:373–378. doi: 10.1002/tera.1420440403. [DOI] [PubMed] [Google Scholar]
- Ervin CS, Little RE, Streissguth AP, Beck DE. Alcoholic fathering and its relation to child’s intellectual development: a pilot investigation. Alcohol Clin Exp Res. 1984;8:362–365. doi: 10.1111/j.1530-0277.1984.tb05681.x. [DOI] [PubMed] [Google Scholar]
- Gabrielli WF, Jr, Mednick SA. Intellectual performance in children of alcoholics. J Nerv Ment Dis. 1983;171:444–447. doi: 10.1097/00005053-198307000-00009. [DOI] [PubMed] [Google Scholar]
- Hallman J, von Knorring L, Oreland L. Personality disorders according to DSM-III-R and thrombocyte monoamine oxidase activity in type 1 and type 2 alcoholics. J Stud Alcohol. 1996;57:155–161. doi: 10.15288/jsa.1996.57.155. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158(2):198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, Fox NC, Rossor AM, Harvey RJ, Rossor MN. Intracranial volume and Alzheimer disease: evidence against the cerebral reserve hypothesis. Arch Neurol. 2000;57:220–224. doi: 10.1001/archneur.57.2.220. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Kolko DJ. Short-term follow-up of child psychiatric hospitalization: clinical description, predictors, and correlates. J Am Acad Child Adolesc Psychiatry. 1992;31:719–727. doi: 10.1097/00004583-199207000-00021. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as morphogens. Prog Brain Res. 1988;73:365–387. doi: 10.1016/S0079-6123(08)60516-6. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Momenan R, Hommer D, Rawlings R, Ruttimann U, Kerich M, Rio D. Intensity-adaptive segmentation of single-echo T1-weighted magnetic resonance images. Human Brain Mapping. 1997;5:194–205. doi: 10.1002/(SICI)1097-0193(1997)5:3<194::AID-HBM4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Perez CM, Widom CS. Childhood victimization and long-term intellectual and academic outcomes. Child Abuse Negl. 1994;18:617–633. doi: 10.1016/0145-2134(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Rosso P. Prenatal nutrition and brain growth. Vol. 58. New York, NY: Wiley-Liss, Inc; 1990. [Google Scholar]
- Sapolsky RM. Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog Brain Res. 1990;86:13–23. doi: 10.1016/s0079-6123(08)63163-5. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Psychological characteristics of children of alcoholics. Overview of research methods and findings. Recent Dev Alcohol. 1991;9:301–326. [PubMed] [Google Scholar]
- Silverstein A. Estimating Full Scale IQs from short forms of Wechsler’s scales: Linear scaling vs. linear regression. J Consult Clin Psychol. 1983;52:199. doi: 10.1037//0022-006x.52.5.919. [DOI] [PubMed] [Google Scholar]
- Silverstein AB. An appraisal of three criteria for evaluating the usefulness of WAIS-R short forms. J Clin Psychol. 1985;41:676–680. doi: 10.1002/1097-4679(198509)41:5<676::aid-jclp2270410515>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Todd RD. Neural development is regulated by classical neurotransmitters: dopamine D2 receptor stimulation enhances neurite outgrowth. Biol Psychiatry. 1992;31:794–807. doi: 10.1016/0006-3223(92)90311-m. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- von Knorring L, Oreland L, von Knorring AL. Personality traits and platelet MAO activity in alcohol and drug abusing teenage boys. Acta Psychiatr Scand. 1987;75:307–314. doi: 10.1111/j.1600-0447.1987.tb02793.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised manual. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- Welch-Carre E. The neurodevelopmental consequences of prenatal alcohol exposure. Adv Neonatal Care. 2005;5:217–229. doi: 10.1016/j.adnc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Willerman L, Schultz R, Rutledge JN, Bigler ED. Brain size and intelligence. Intelligence. 1991;15:223–228. [Google Scholar]


