Abstract
Objectives
Patients with a missense mutation of the calsequestrin 2 gene (CASQ2) are at risk for catecholaminergic polymorphic ventricular tachycardia. This mutation (CASQ2 D307H) results in decreased ability of CASQ2 to bind Ca2+ in the sarcoplasmic reticulum (SR). In this theoretical study, we investigate a potential mechanism by which CASQ2D307H manifests its pro-arrhythmic consequences in patients.
Methods
Using simulations in a model of the guinea pig ventricular myocyte, we investigate the mutation's effect on SR Ca2+ storage, the Ca2+ transient (CaT), and its indirect effect on ionic currents and membrane potential. We model the effects of isoproterenol (ISO) on CaV1.2 (the L-type Ca2+ current, ICa(L)) and other targets of β-adrenergic stimulation.
Results
ISO increases ICa(L), prolonging action potential (AP) duration (Control: 172 ms, +ISO: 207 ms, at cycle length of 1500 ms) and increasing CaT (Control: 0.79 μM, +ISO: 1.61 μM). ISO increases ICa(L) by reducing the fraction of channels which undergo voltage-dependent inactivation and increasing transitions from a non-conducting to conducting mode of channel gating. CASQ2 D307H reduces SR storage capacity, thereby reducing the magnitude of CaT (Control: 0.79 μM, CASQ2 D307H: 0.52 μM, at cycle length of 1500 ms). The combined effect of CASQ2 D307H and ISO elevates SR free Ca2+ at a rapid rate, leading to store-overload induced Ca2+ release and delayed afterdepolarizations (DAD). If resting membrane potential is sufficiently elevated, the Na+-Ca2+ exchange-driven DAD can trigger INa and ICa(L) activation, generating a triggered arrhythmogenic AP.
Conclusions
The CASQ2D307H mutation manifests its pro-arrhythmic consequences due to store-overload-induced Ca2+ release and DAD formation due to excess free SR Ca2+ following rapid pacing and β-adrenergic stimulation.
Introduction
Calsequestrin (CASQ2) is a high-capacity Ca2+ binding protein in the sarcoplasmic reticulum (SR) of cardiac myocytes [1]. Its role is to store Ca2+ taken into the SR between contractions, making available a large quantity of Ca2+ for release upon opening of SR Ca2+ release channels (RyRs). Patients with homozygous expression for the missense mutation CASQ2D307H to a highly conserved region of the CASQ2 gene are at risk of developing catecholaminergic polymorphic ventricular tachycardia (CPVT) which can deteriorate to fibrillation and sudden death [2]. Patients are at greatest risk during exercise, emotional distress, or other periods of elevated β-adrenergic tone. It is unclear how the mutation changes CASQ2's ability to bind Ca2+. Studies in rat ventricular myocytes [3] expressing CASQ2D307H show that total Ca2+ binding capacity is reduced by approximately 60%, resulting in smaller Ca2+ transients. In the presence of isoproterenol (ISO), increased occurrence of spontaneous SR Ca2+ release, (store-overload-induced Ca2+ release (SOICR)) [4] is observed. A recent study showed that CASQ2D307H alters CASQ2 sensitivity to Ca2+ and its interaction with RyR linking proteins, junctin and triadin [5].
Here we simulate the effects of the CASQ2D307H mutation and explore SOICR as a possible trigger mechanism for CPVT utilizing a mathematical (computer) model of the mammalian ventricular myocyte. Because CPVT occurs during β-adrenergic stimulation, we incorporate changes to the L-type Ca2+ current (ICa(L)) and other currents, reflecting the application of a saturating concentration of ISO (≥0.1 μM). Parts of this work were previously published in abstract format [6].
Methods
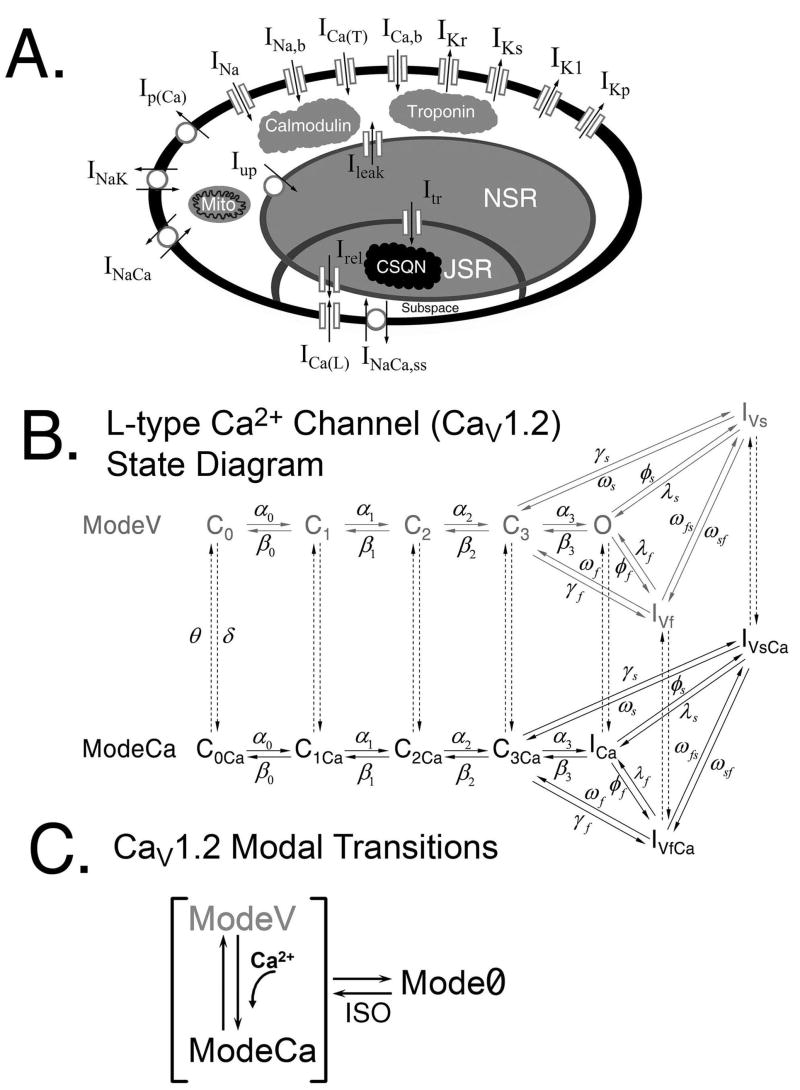
The theoretical LRd model of a guinea pig ventricular action potential (AP) (Fig. 1A) provides the basis for the simulations in this study [7, 8]. We recently developed a detailed, structure-based Markov model of the L-type Ca2+ channel (CaV1.2) and incorporated it into the LRd model cell, where it interacts with a Markov model of RyR in a restricted t-tubular subsarcollemal space for Ca2+ distribution (Fig. 1A) [8]. Intracellular Ca2+ cycling processes represented in this model include Ca2+ uptake and release by the SR and its buffering. Buffers include calmodulin and troponin (in the myoplasm), sarcolemmal and SR Ca2+ binding sites (in the t-tubules subsarcolemmal space), and calsequestrin (in the SR). The model of reference [8] is used in the studies presented here, with the following modifications: 1) Time dependent uptake and release of Ca2+ by mitochondria in the myoplasm. 2) Changes to model parameters that reflect the addition of a saturating concentration of ISO (≥0.1 μM). In addition to ISO effects on ICa(L), we incorporate its effects on the following processes: the slow delayed rectifier K+ current (IKs), the Na+-K+ pump (INaK), the inwardly rectifying K+ current (IK1), and the SR Ca2+ ATPase (Iup). 3) Modification of the CaV1.2 kinetic model (Fig. 1B) to include an additional non-conducting gating mode (Mode0, Fig. 1C). 4) Modification of the RyR model that allows for spontaneous release of SR Ca2+. 5) Changes to the CASQ2 formulation to represent mutant CASQ2D307H in simulations of the mutation. Details of these modifications, model equations, and parameter definitions are provided in the Online Supplement.
FIGURE 1.
(A) Schematic of the five compartment myocyte model (bulk myoplasm, JSR – junctional SR, NSR – network SR, mito – mitochondria, and t-tubular subspace). (B) State diagram of the L-type Ca2+ channel Markov model. The conducting mode, ModeV (grey) consists of four closed states (C0, C1, C2, and C3), a single conducting state (O), an inactivation state into which channels move rapidly (IVf) following depolarization, and an inactivation state into which channels enter more slowly (IVs). ModeCa (black) is a non-conducting mode representing channels which have inactivated due to Ca2+. (C) Modal transitions of the CaV1.2 Markov model. From any of the states in ModeV, channels may inactivate via CDI (shown as a transition from ModeV to ModeCa). Channels can also transition into Mode0, a non-conducting mode that serves as a reserve of channels that are activated in the presence of ISO. Details are in the reference [8] and Online Supplement.
Results
Effects of Isoproterenol on ICa(L)
Favorable conditions for CPVT initiation occur during episodes of elevated β-adrenergic stimulation, which has been simulated experimentally by ISO application. Here we conduct theoretical simulations of the effects of ISO on cellular processes. ISO has a significant effect on AP duration (APD) and morphology and increases myocyte contractility. Of greatest significance to this study are its effects on CaV1.2 due to the role of ICa(L) as 1) the primary trigger of CICR, 2) a major determinant of APD, and 3) a major source of Ca2+ entry and SR loading. The application of ISO to CaV1.2 results in several important changes to the behavior of the channel, which are summarized below and depicted in Fig. 2, Fig. 3, and Table 1.
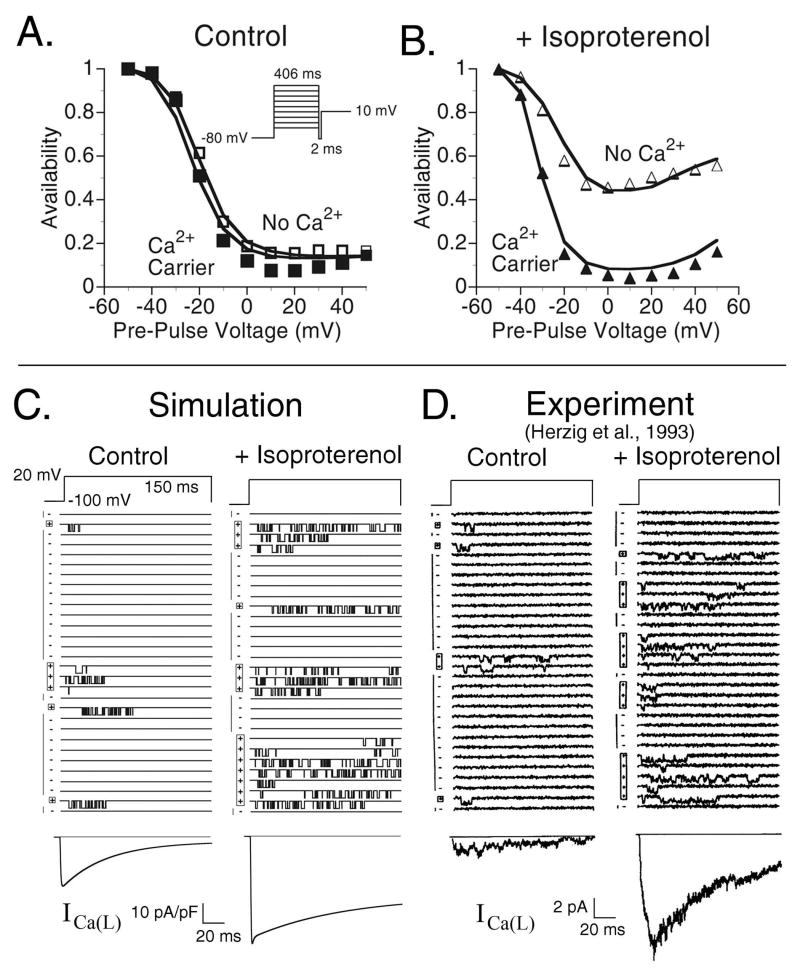
FIGURE 2.
(A) ICa(L) steady-state inactivation curves during control conditions. Simulations (line traces) are compared to experimental data from Findlay (symbols) [10] for conditions where Ca2+ is not the charge carrier (□) (VDI) and where Ca2+ is the charge carrier (■) (VDI + CDI). (B) CaV1.2 steady state inactivation curves following the application of 0.1 μM isoproternol. As in Panel A, the line traces are simulated curves and the symbols are experimental data [10] for conditions where Ca2+ is not the charge carrier (△) and where Ca2+ is the charge carrier (▲). The protocol for both Panels A and B is shown in the inset of Panel A. The experimental data were measured at room temperature and the simulations conducted at 37°C; times shown in the inset are the experimental times adjusted to 37°C utilizing a Q10=2. In the experiments, the non-Ca2+ charge carrier was Mg2+, which we simulate by eliminating CDI in the model. The experimental data were recorded with ryanodine in the solution, simulated here by setting Grel = 0. (C) CaV1.2 single channel traces generated using the model in the absence of CDI. The voltage is clamped to 20 mV from a holding potential of −100 mV for a duration of 150 ms every 600 ms. Left column: model results during control conditions and right column: model results following application of ISO. The ensemble (whole cell) current is shown below the single channel traces. (D) Experimentally measured single channel traces [12] generated utilizing the same protocol as Panel A. The ensemble current is shown below the single channel traces.
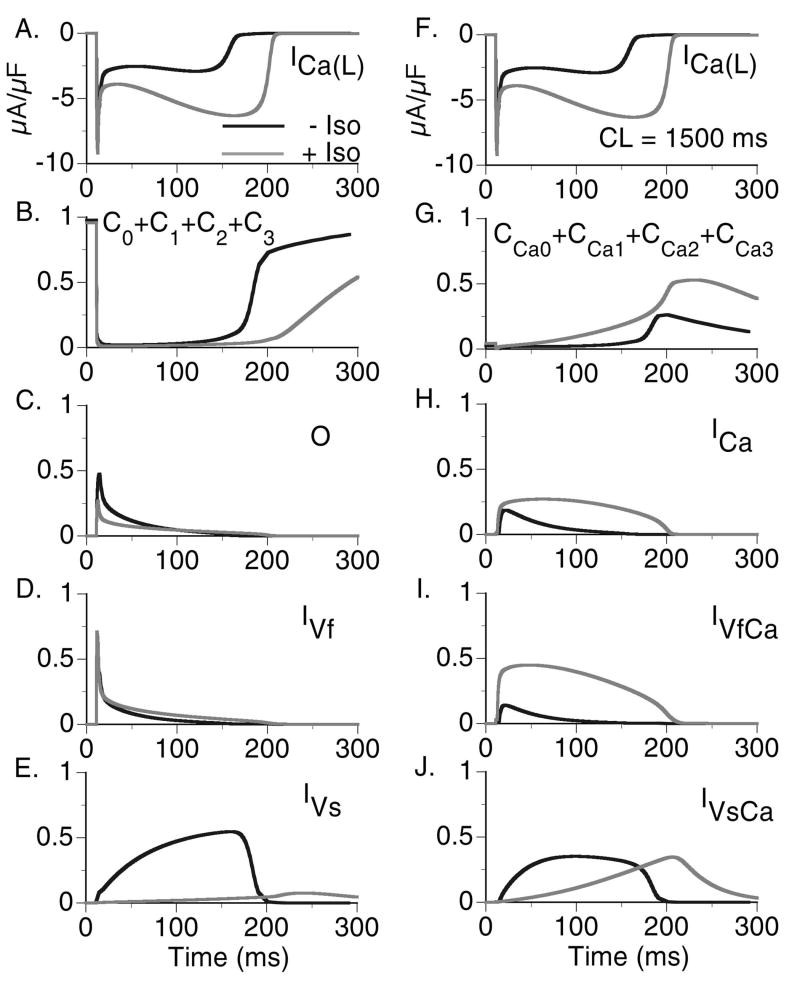
FIGURE 3.
State residencies of the L-type Ca2+ channel during the time course of the action potential at CL=1500 ms in the absence of ISO (black curve) and in the presence of ISO (grey curve). For identification of the channel states, refer to Fig. 1B.
Table 1.
Comparison of Single Channel Gating Properties
| Experiment
(Herzig, et al. [12]) |
Simulation | |||
|---|---|---|---|---|
| Control | Isoproterenol | Control | Isoproterenol | |
| Slow Gating | ||||
| Active sweeps (% of total) | 22.9 ± 4.8% | 53.3 ± 9.9% | 24% | 56.5% |
| Active run lifetime (s) | 1.3 ± 0.3 | 3.4 ± 1.1 | 1.7 | 3.14 |
| Blank run lifetime (s) | 5.0 ± 1.2 | 2.2 ± 0.7 | 5.3 | 2.4 |
|
|
||||
| Fast Gating | ||||
| Mean open time (ms) | 1.0 ± 0.0 | 1.1 ± 1.1 | 1.0 | 1.03 |
| Mean closed time (ms) | 4.1 ± 0.7 | 2.2 ± 0.2 | 5.81 | 3.05 |
CaV1.2 channels inactivate via two different mechanisms, voltage-dependent inactivation (VDI) and Ca2+-dependent inactivation (CDI). Our model of CaV1.2 assumes that these means of inactivation are not mutually exclusive and that the channel can exist in a state where it is inactivated via both mechanisms. In a previous publication [8], we showed how the interaction of these two mechanisms of inactivation determines the morphology of ICa(L), the [Ca2+]i transient (CaT), and the AP in the absence of ISO. A series of studies by Findlay [9, 10], showed that ISO alters the mechanism of inactivation that dominates total inactivation of the channel, switching from VDI dominant without ISO to CDI dominant following the application of ISO. Fig. 2A and 2B shows steady state inactivation curves for CaV1.2 generated by the model (solid curves) versus experimental data (symbols) [10]. Panel 2A shows control conditions without ISO and Panel 2B shows conditions following the application of 0.1 μM ISO. In the absence of CDI (Mg2+ is the charge carrier in the experiment) VDI is greatly reduced in the presence of ISO (Fig. 2B, △). When Ca2+ is the charge carrier (meaning that channels can now inactivate via CDI), total steady-state inactivation in the presence of ISO (Fig. 2B, ▲) is greatly increased towards levels of inactivation in the control (Fig. 2A). The experiments were conducted in the presence of 5 μM ryanodine which we simulate by blocking SR Ca2+ release.
In addition to the changes to inactivation of ICa(L), ISO increases the magnitude of the current. Several studies have shown that this is due to the transition of a significant percentage of channels from a non-conducting mode (Mode0) into the normal mode (often referred to as Mode1; ModeV in our model) where they become available to open upon depolarization [11, 12]. In single channel recordings, Mode0 is represented in blank sweeps. Fig. 2C shows simulated single channel recordings compared to experimentally measured single channel recordings (Fig. 2D) in the absence of CDI (Mg2+ as the charge carrier). In both simulation and experiment [12], the traces are measured sequentially during a voltage step from −100 mV to 20 mV for 150 ms applied every 600 ms. Single channel statistical data, including the percentage of active sweeps, the active run lifetime, blank run lifetime, mean open time, and mean closed time are summarized in Table 1, which provides a quantitative comparison between model and experiment. Following the application of ISO (0.8 μM in the experiment) the number of active sweeps increases in both the model simulation (Fig. 2C, right) and experiment (Fig. 2D, right) compared to control. In addition, the number of openings during an active sweep increases significantly due to the reduction in VDI, with no change to channel mean open time [11, 12]. The ensemble currents (shown below in Fig. 2C and D) clearly show the increase in current magnitude.
Effects of Isoproterenol on ICa(L) State Residencies
In Fig. 3 a comparison between the control ICa(L) current and the current following ISO application is shown with corresponding channel state residencies for CL=1500 ms. In the presence of ISO, VDI is greatly reduced (Fig. 3E) and the percentage of channels which undergo CDI is greatly increased (Fig. 3H and 3I). The morphology of ICa(L) is changed by ISO, showing much greater dependence on the time course of the CaT (see Fig. 4A), a reflection of the increased dependence of ICa(L) on CDI rather than VDI. Note that the Open probability in the presence of ISO is less than that in the Control. It should be noted that this is different from the behavior observed in the absence of CDI as shown in Fig. 2C and 2D where the addition of ISO increased channel Open probability. Despite the reduction in Open probability, ICa(L) is still larger in the presence of ISO than control conditions due to the shift of channels from the non-conducting Mode0 to ModeV. CaV1.2 channel state residencies with and without ISO for a more rapid pacing rate (CL=500 ms) are provided in the Online Supplement Fig. E1.
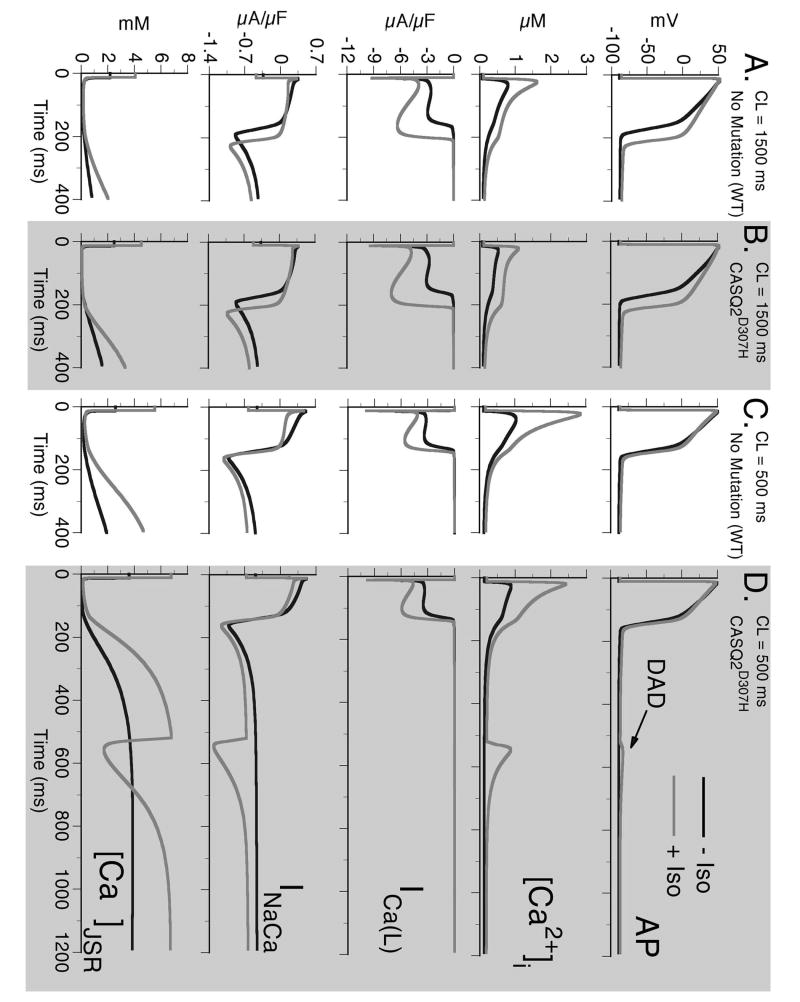
FIGURE 4.
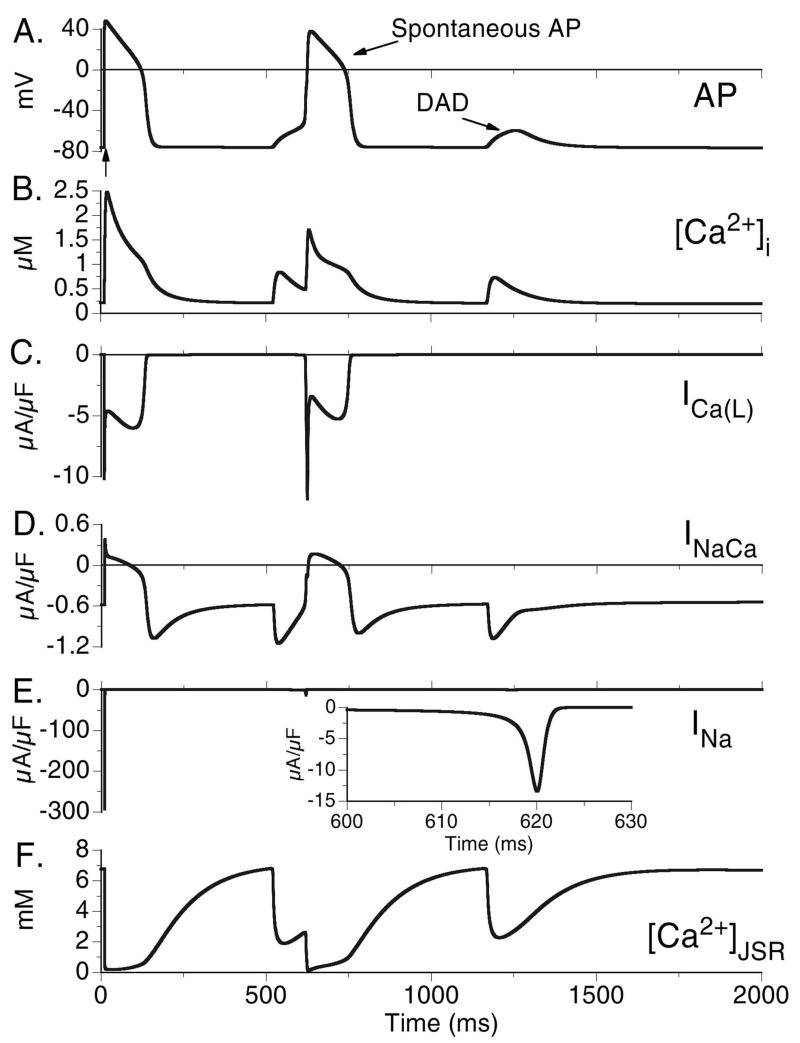
Top to bottom: Action potential (AP), calcium transient ([Ca2+]i), L-type Ca2+ current (ICa(L)), Na+-Ca2+ exchange current (INaCa), and free JSR Ca2+ ([Ca2+]JSR) for combinations of six different protocols: 1) slow pacing (CL=1500 ms) - Panels A and B. 2) fast pacing (CL=500 ms) – Panels C and D. 3) Wild type (WT) – non-shaded columns. 4) CASQ2D307H – shaded columns. 5) Without Isoproterenol (−ISO) – black traces. 6) With Isoproterenol (+ISO) – grey traces. In all panels, the last paced beat is shown after pacing for 5 minutes at the indicated cycle length followed by a period where no stimulus is applied. Note that only the combination of fast rate, CASQ2D307H mutation, and +ISO results in generation of delayed afterdepolarization (DAD) after cessation of pacing (arrow in Panel D). The DAD is generated when [Ca2+]JSR reaches a threshold concentration, which results in opening of RyRs and spontaneous Ca2+ release. The resulting rise in [Ca2+]i leads to increased depolarizing INaCa current as it removes the excess Ca2+.
Effects of ISO on the Wild-type (WT) AP
A comparison between the control myocyte and the myocyte with ISO application at a cycle length of 1500 ms is shown in Fig. 4A. The ISO-dependent reduced inward rectification of IK1 at negative potentials results in an elevation of the resting membrane potential (RMP) by 2 mV and, at this cycle length, ISO prolongs APD90 by 36 ms from 172 ms to 207 ms. This is in close agreement with experimental studies by Rocchetti et al. [13] who observed a prolongation of APD90 from 176±10 ms to 220±10 ms following the application of 0.1 μM ISO. The cause of APD prolongation is due to a greatly increased ICa(L) during the AP plateau. In our simulations, CDI of ICa(L) is a function of subspace Ca2+ ([Ca2+]ss, not shown) but the correlation is clear, as CaT declines so does [Ca2+]ss and ICa(L) recovers from CDI.
ISO has a strong positive inotropic effect, increasing the magnitude of CaT from 0.79 μM to 1.61 μM as well as increasing the amount of Ca2+ in the JSR that is available for release. This is due mainly to increased Ca2+ entry via ICa(L) leading to Ca2+ loading of the myocyte. A summary of ISO effects on APD90, RMP, peak [Ca2+]i, and diastolic [Ca2+]i at a CL=1500 ms is provided in Table 2. In addition, Table 2 indicates the isolated effect of each ISO-induced change on these parameters. The changes to ICa(L) almost fully account for the increase in APD and the elevation of CaT. Increased IKs counteracts the APD prolongation due to ICa(L) and this also leads to a decrease in [Ca2+]i indirectly, by increasing the diastolic interval during which INaCa can remove excess Ca2+. The increase in INaK also counteracts [Ca2+]i accumulation by reducing [Na+]i thereby allowing for efficient removal of [Ca2+]i via INaCa. The elevation of the RMP is due solely to the decrease in IK1, a result of the decreased inward rectification of the current in the presence of ISO.
Table 2.
Comparison of Isolated ISO Effects on APD90, RMP, Peak and Diastolic [Ca2+]i
| CL = 1500 ms | Control | +ISO | ICa(L) Changes Only | Iup Changes Only | IK1 Changes Only | IKs Changes Only | INaK Changes Only |
|---|---|---|---|---|---|---|---|
| APD90
(ms) |
172 | 207 | 266 | 172 | 174 | 143 | 176 |
|
|
|||||||
| RMP
(mV) |
−88.9 | −86.1 | −88.8 | −88.8 | −86.1 | −89.0 | −89.3 |
|
|
|||||||
| Peak [Ca2+]i
(μM) |
0.80 | 1.65 | 2.22 | 0.871 | 0.794 | 0.77 | 0.621 |
|
|
|||||||
| Diastolic [Ca2+]i
(μM) |
0.070 | 0.105 | 0.134 | 0.073 | 0.072 | 0.067 | 0.057 |
As pacing frequency increases, the differences in APD between control and ISO decrease (Fig. 4C). This behavior is also in agreement with experiments [13] and results from decreased ICa(L) due to increased CDI (secondary to increased Ca2+ loading) and increased IKs due to current accumulation at fast rates [13-15].
CASQ2 Mutation and Triggered Activity
Fig. 4 depicts eight conditions, only one of which results in spontaneous triggered activity. Panels A and B show pacing at a slow rate (CL=1500 ms) and Panels C and D show pacing at a rapid rate (CL=500 ms). Panels A and C show WT behavior and Panels B and D are results for the CASQ2D307H mutation. In each panel, the black curves are simulations with no ISO and the grey curves are conditions where ISO is present. Conditions for delayed afterdepolarization (DAD) generation (Fig. 4D) require rapid pacing and ISO application (both leading to increased Ca2+ loading) in the CASQ2D307H mutant cell. The mutation decreases Ca2+ buffering in the SR, leading to excess free Ca2+ in the JSR (Fig. 4D, [Ca2+]JSR). Despite excess free [Ca2+]JSR, the total JSR Ca2+ (free [Ca2+]JSR + Ca2+ bound to CASQ2) is much less (total JSR Ca2+, CL=1500 ms, diastolic values: WT=9.5 mM; CASQ2D307H=5.5 mM; WT+ISO=12.4 mM; CASQ2D307H + ISO=7.9 mM), leading to smaller CaT compared to WT (Peak [Ca2+]i, CL=1500 ms: WT=0.79 μM; CASQ2D307H=0.52 μM). A summary of the effects of reduced CASQ2 buffering capacity on the CaT, free Ca2+ in the JSR, and total Ca2+ in the JSR are provided in the Online Supplement Fig. E3.
Upon exceeding its Ca2+ storage capacity, the SR spontaneously releases its Ca2+ stores (Fig. 4D, [Ca2+]JSR), the subsequent rise in [Ca2+]i is removed by the Na+-Ca2+ exchanger. Being an electrogenic exchanger, it generates an inward current (Fig. 4D, INaCa) which depolarizes the membrane to generate a DAD. Note that ICa(L) does not participate in the DAD generation (Fig. 4D, ICa(L)).
For conditions where the RMP is further depolarized (hypokalemia, K+ channel blocking drugs, heart failure [16]), the Na+-Ca2+ exchange driven DAD can be of sufficient magnitude to activate INa and ICa(L), leading to the generation of a spontaneous AP. In Fig. 5 conditions are identical to those in Fig. 4D except the RMP is elevated by reducing IK1. This elevation in RMP creates conditions whereby the DAD is able to activate INa (Fig 5E) and ICa(L) (Fig 5C) to trigger a spontaneous AP (Fig 5A). The second overload-induced SR Ca2+ release generates a DAD, but the depolarizing current generated by INaCa (Fig. 5D) is no longer sufficient to trigger a fully-developed AP.
FIGURE 5.
DAD that triggers a spontaneous AP. Conditions are the same as Fig. 4D that resulted in DAD formation (CL=500 ms, +CASQ2D307H mutation, +ISO) with additional reduction of IK1. The arrow in Panel A indicates the last paced beat which is followed by a DAD that generates a spontaneous AP. Due to the elevated RMP and increased excitability (due to smaller IK1), the depolarization is large enough to trigger activation of both INa (Panel E) and ICa(L) (Panel C) leading to the generation of an AP. A second spontaneous Ca2+ release event also occurs, but does not generate sufficient depolarizing current to generate a triggered AP.
Pro-arrhythmic Consequences of a Pause During Pacing
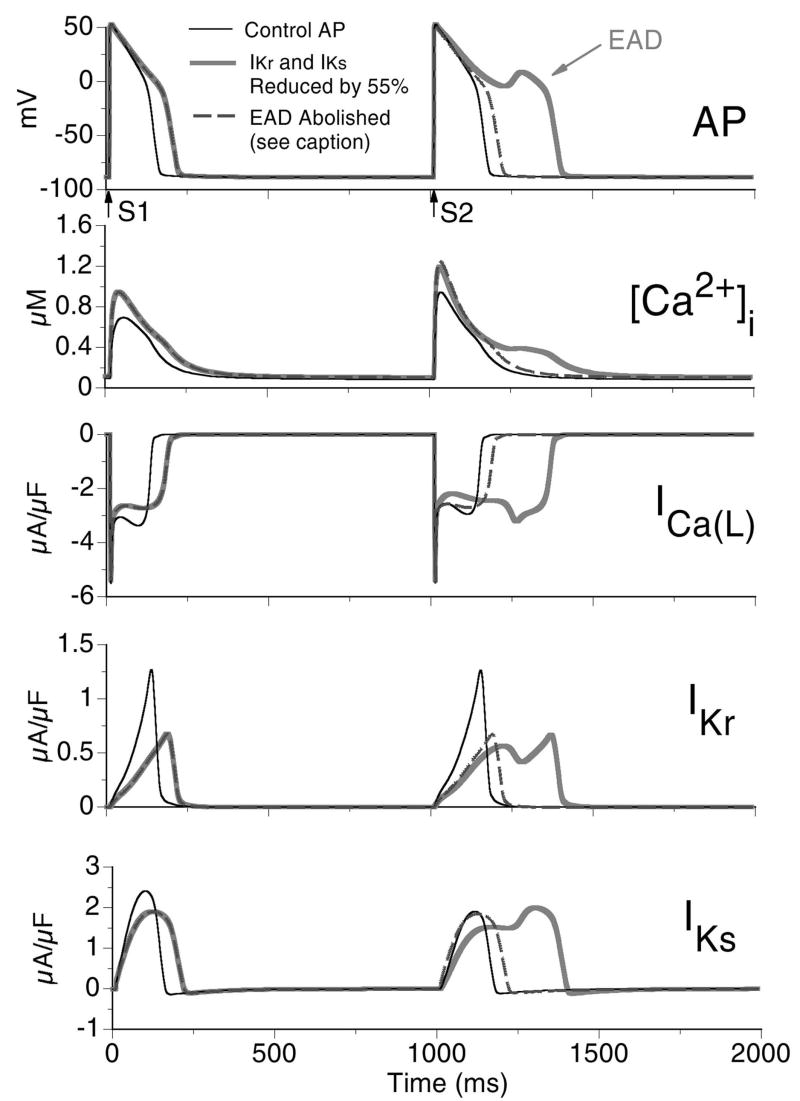
We demonstrated that cessation or pause of fast pacing could lead to elevated Ca2+ within the SR which, on the background of ISO and CASQ2D307H, was sufficiently increased to result in SOICR and the generation of a DAD. The pause was of great significance in the DAD development in that it allowed time for free Ca2+ in the JSR to reach a level high enough for SOICR to occur. Another arrhythmia-triggering mechanism which may arise as the result of a pause during pacing is the generation of early afterdepolarizations (EAD). While EAD generation does not require conditions associated with CPVT, it is of mechanistic interest to compare these two pause-dependent arrhythmogenic phenomena. In Fig. 6 we simulate pause-induced EAD generation using the cell model and the CaV1.2 Markov model. In the simulation protocol [17], IKr and IKs are reduced by 55% in the wild-type (WT) cell (no ISO and no CASQ2D307H mutation) and the myocyte is paced for 40 beats at a CL=500 ms followed by a 1000 ms pause before the next stimulus. In Fig. 6C it can be observed that following the pause, the next paced beat exhibits reactivation of ICa(L) (Fig. 6C, arrow) that depolarizes the membrane potential to generate the EAD (Fig. 6A, arrow). This occurs on the background of reduced repolarizing IKs during the post-pause AP (Fig. 6E) due to greater deactivation during the long pause. Preventing recovery from inactivation of ICa(L) (either recovery from VDI or CDI, see Online Supplement Fig. E3) can effectively abolish the EAD. Similarly, increasing repolarizing current (and hence shortening APD) is also effective at abolishing the EAD as shown in Fig. 6 (dashed curve), where the IKs gates prior to the S2 stimulus are reset to pre-pause values prior to S1. The Markov model of ICa(L) provides new insight into the mechanism of EAD formation at the level of transitions between channel kinetic states.
FIGURE 6.
Pause induced early afterdepolarization (EAD). The myocyte is paced for 40 beats at CL=500 ms (S1) followed by a 1000 ms pause before the next stimulus (S2). Panel A: Last paced and the post-pause APs; arrows indicate time of stimuli. Thin black trace shows control AP (pre-pause APD90=151 ms, post-pause APD90=168 ms); thick grey trace shows AP for 55% reduction of IKr and IKs (pre-pause APD90=243 ms, post-pause AP develops EAD and APD90=424 ms); dashed trace shows that the EAD is abolished by resetting IKs to its value at S1, thus preventing additional IKs deactivation during the long pause (pre-pause APD90=243 ms, post-pause APD90=229 ms). Panel B: Corresponding [Ca2+]i. Panels C, D, and E: ICa(L), IKr, and IKs, respectively.
Discussion
In this paper we present simulations which indicate a possible mechanism for the initiation of CVPT in patients with homozygous expression of the CASQ2 mutation D307H. A combination of the conditions of rapid heart rate and β-adrenergic stimulation leads to increased myocyte Ca2+ loading which culminates in spontaneous SR Ca2+ release due to decreased Ca2+ binding by CASQ2D307H and an excess of free [Ca2+]JSR. This is in agreement with observations in patients with the CASQ2D307H mutation who experience the onset of CVPT during exercise or heightened emotional distress. This mechanism for the initiation of CVPT has been proposed by others [2, 3] and studies utilizing rat myocytes expressing CASQ2D307H have demonstrated an increased propensity for SOICR events following the application of ISO [3]. Utilizing a model of the ventricular myocyte, we determine the combination of factors that result in a SOICR event and its effect on the membrane potential. We identify the underlying currents that cause membrane depolarization (DAD) and explore conditions for which the DAD generates a spontaneous AP. In summary, the important findings are: 1) ISO increases ICa(L) current magnitude via two mechanisms; a reduction in the fraction of channels which undergo VDI and an increase in the number of channels which move from a non-conducting mode (Mode0) into the normal mode (ModeV). 2) ISO mediated increase of ICa(L) leads to APD prolongation and increased Ca2+ entry into the myocyte. This, combined with increased SR Ca2+ uptake, leads to elevated SR Ca2+ and a larger CaT. 3) CASQ2D307H reduces the magnitude of CaT by reducing the buffering capacity of CASQ2 and thus causing a reduction in total JSR Ca2+. 4) A SOICR event and triggered DAD is observed when the following three conditions are combined: rapid pacing (which increases mycoyte Ca2+), +ISO (also increases myocyte Ca2+), +CASQ2D307H (which reduces CASQ2 binding of Ca2+ and increases free Ca2+ in JSR). 5) The DAD is generated by the electrogenic Na+-Ca2+ exchange current removing excess Ca2+ released during the SOICR event. 6) Further elevation of the RMP (e.g., by reduction of IK1) allows for the DAD to activate Na+ and L-type Ca2+ channels, leading to the generation of a spontaneous AP.
In addition, as a comparative simulation we present another mechanism of triggered activity, the EAD, that results from reactivation of the CaV1.2 following prolongation of the AP. We study the mechanism of EAD formation at the level of transitions between channel kinetic states. This provides additional insight as to the role of recovery from VDI and CDI in triggered activity and arrhythmogenesis.
CASQ2 Modeling Limitations
There are three potential ways in which the CASQ2D307H mutation may lead to the reduced SR storage capacity of Ca2+ and increased propensity for SOICR events. The first is perhaps the most obvious and is the mechanism which we have chosen to model in this study. We reduced the amount of Ca2+ which CASQ2D307H was able to sequester, which led to a decrease in the SR capability to store Ca2+ and a reduced CaT. It also led to increased free Ca2+ in the JSR, promoting the development of SOICR and DADs.
Other potential ways in which the mutation may change CASQ2 function and result in similar arrhythmogenic behavior include 1) a decrease in the rate at which Ca2+ binds to CASQ2 or 2) modification of the way CASQ2 modulates RyR gating via junctin and triadin. The first is effectively equivalent to the reduced buffering capacity simulated here. The second requires additional experimental information, describing exactly how CASQ2 modulates RyR activity and how this interaction is modified by the mutation, before a more detailed model can be developed. It would also allow for model study of a recently discovered CASQ2 mutation, CASQ2R33Q, that also results in CVPT [18]. This mutation, when expressed in adult rat myocytes via adenoviral transfection, does not exhibit reduced Ca2+ binding capacity like CASQ2D307H, but does show increased occurrence of SOICR in the presence of ISO.
β-adrenergic Effect on CDI and VDI
In a report by Findlay [19], he hypothesizes that β-adrenergic modulation of ICa(L) inactivation occurs via a ‘switch’ mechanism. To summarize, in the absence of ISO, fast VDI is present and channels which inactivate via this mechanism cannot be inactivated by CDI. When ISO is present, fast VDI is switched off and CDI becomes the predominant mechanism of channel inactivation. It is important to note that our simulations do not fit this theory of complete switching in that our Markov representation allows for channels to be simultaneously inactivated via CDI and VDI. However, consistent with the switching concept, when ISO is present in the simulations CDI becomes the major mechanism of channel inactivation, although fast VDI still contributes to the inactivation process.
CaV1.2 Modal Gating
Single channel recordings in the presence of ISO in experiments conducted by Yue et al. [20] show an increased percentage of channels shifting from Mode0 into the normal mode (which they refer to as Mode1), as we show in Fig. 3. However, Yue et al. also observed a percentage of channels shifting into an additional mode (referred to as Mode2) that is characterized by open times that are much longer than those in the normal mode. We do not include this mode in our Markov representation of CaV1.2 to prevent over complexity of the model and due to the fact that an increase of Mode2 gating was not observed in other experiments [11, 12]. Results from Tsien et al. [11] and Herzig et al. [12] show that ISO does not have a significant effect on mean open time. If there had been an increase in Mode2 gating in their studies, they would have observed increase in mean open time. A possible explanation for the observed Mode2 gating in Yue et al. experiments is that the measurements were conducted at room temperature, which allowed for channels to remain in Mode2 that is short lived at body temperature (the temperature at which our model simulations have been conducted).
Inter-species Difference in AP Morphology and ICa(L)
The simulations conducted in this study utilize a model of the mammalian ventricular mycoyte based primarily on data from the guinea pig. There are inter-species differences in channel density and kinetics, the most prominent being the lack of the transient outward K+ current (Ito) in guinea pig ventricular myocytes. This current is present in both human and canine myocytes and is responsible for the spike and dome morphology of the epicardial AP (and to a lesser degree, the mid-myocardial AP) in these species. Despite these differences, the mechanism of DAD development simulated here is model independent due to the fact that increased ICa(L) and Ca2+ accumulation within the myocyte are observed in all species following the application of ISO. We verified this by repeating the simulations utilizing a modified model of the guinea pig cell to which we added Ito based upon the formulation of Dumaine et al. [21] (data not shown) and obtained qualitatively similar results to those reported here.
The addition of Ito lowers the AP plateau which increases the driving force for ICa(L). This, in turn, increases the magnitude of the current and the amount of Ca2+ entry. It is possible that this increased amount of Ca2+ could increase the likelihood of SOICR in human myocytes under conditions of ISO in presence of the CASQ2D307H mutation.
In addition to differences in AP morphology, there are also differences in ICa(L) properties between species, including variation in channel density, kinetics, and modulation by accessory subunits. In a series of studies by Findlay in guinea pig myocytes [9, 10, 19], upon which the VDI kinetics of our model are based, he demonstrated that VDI contributes significantly to total ICa(L) inactivation compared to CDI in the absence of ISO. However, it is important to note that Findlay's studies were conducted in the absence of SR Ca2+ release. In a previous publication [8], we have shown that simulations of ICa(L) with SR release intact results in approximately 50% of channels inactivating via CDI, a value comparable to that observed in rat [22] and rabbit [23]. This suggests that differences observed as to the contribution of CDI to total ICa(L) inactivation depend largely on experimental protocol (i.e. block vs. no block of SR release) rather than on intrinsic differences between species.
Chloride Channels
In our model of the guinea pig ventricular myocyte, we do not include chloride channels, nor do we keep track of dynamic changes in intracellular chloride ([Cl−]i). Two Cl− channels which may play a role during β-adrenergic stimulation and conditions of Ca2+-overload are the β-adrenergically modulated Cl− channel (ICl,cAMP) and the Ca2+ activated Cl− channel (ICl(Ca)). ICl(Ca), although implicated as an important contributor to DAD formation in canine, rabbit and sheep myocytes, is not found in either guinea pig or human ventricular myocytes [24], thus its inclusion would not alter the results presented here, nor their implications to clinical arrhythmogensis.
ICl,cAMP is activated by the stimulation of β-adrenergic receptors and has been shown to participate in regulation of cell volume and as a modulator of APD and RMP [25]. At hyperpolarized potentials (Vm<−50 mV), ICl,cAMP is depolarizing and can lead to slight elevation of RMP. At Vm>−50 mV, the current is repolarizing and leads to significant APD shortening. This has important implications to our study since APD prolongation following β-adrenegic stimulation, due to increased ICa(L), is important for Ca2+ loading of the myocyte and reducing the diastolic interval during which Ca2+ can be removed. We have shown that the simulated ISO induced APD changes are similar to those observed by Rocchetti et al. [13]. In simulations where we add a repolarizing current with characteristics of ICl,cAMP (Erev=−50 mV, linear dependence on voltage with a current magnitude of 3 uA/uF at 50 mV, and time independence) and then either decrease INaK or increase ICa(L) to achieve the experimentally measured APD prolongation of 50 ms, [Ca2+]i increases to a level even greater than that during ISO simulations without ICl,cAMP, thus leaving the conclusions unaltered (i.e. loading of the myocyte with Ca2+ along with lowered CASQ2 buffering capacity leads to SOICR and DAD development). In simulations where we add the same repolarizing current, but decrease IKs to achieve APD prolongation of 50 ms, the [Ca2+]i levels are similar to those in the simulations of ISO without ICl,cAMP, and the conclusions remain unchanged.
Supplementary Material
Acknowledgments
This research was supported by NIH-NHBLI Merit Award R37-HL33343 and grant RO1-HL49054. Yoram Rudy is the Fred Saigh Distinguished Professor at Washington University. Thanks to our laboratory members Leonid Livshitz, Keith Decker, Tom O'Hara, Namit Gaur, and Ali Nekouzadeh and our computer administrator Li Li.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–84. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viatchenko-Karpinski S, Terentyev D, Györke I, Terentyeva R, Volpe P, Priori SG, et al. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ Res. 2004;94:471–7. doi: 10.1161/01.RES.0000115944.10681.EB. [DOI] [PubMed] [Google Scholar]
- 4.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci USA. 2004;101:13062–7. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houle TD, Ram ML, Cala SE. Calsequestrin mutant D307H exhibits depressed binding to its protein targets and a depressed response to calcium. Cardiovasc Res. 2004;64:227–33. doi: 10.1016/j.cardiores.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Faber GM, Rudy Y. Calsequestrin Mutation Results in Spontaneous Calcium Release and Delayed Afterdepolarizations in a Model of the Cardiac Ventricular Myocyte. Circulation. 2005;112:II–17. Abstract. [Google Scholar]
- 7.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–96. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 8.Faber GM, Silva J, Livshitz L, Rudy Y. Kinetic Properties of the Cardiac L-type Ca2+ Channel and its Role in Myocyte Electrophysiology: A Theoretical Investigation. Biophys J. 2007;92:1522–43. doi: 10.1529/biophysj.106.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Findlay I. Beta-adrenergic and muscarinic agonists modulate inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J Physiol. 2002;545:375–88. doi: 10.1113/jphysiol.2002.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Findlay I. beta-Adrenergic stimulation modulates Ca2+- and voltage-dependent inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J Physiol. 2002;541:741–51. doi: 10.1113/jphysiol.2002.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- 12.Herzig S, Patil P, Neumann J, Staschen CM, Yue DT. Mechanisms of beta-adrenergic stimulation of cardiac Ca2+ channels revealed by discrete-time Markov analysis of slow gating. Biophys J. 1993;65:1599–612. doi: 10.1016/S0006-3495(93)81199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocchetti M, Freli V, Perego V, Altomare C, Mostacciuolo G, Zaza A. Rate dependency of {beta}-adrenergic modulation of repolarizing currents in the guinea-pig ventricle. J Physiol. 2006;574:183–93. doi: 10.1113/jphysiol.2006.105015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faber GM, Rudy Y. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J. 2000;78:2392–404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva J, Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005;112:1384–91. doi: 10.1161/CIRCULATIONAHA.105.543306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–67. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 17.Viswanathan PC, Rudy Y. Pause induced early afterdepolarizations in the long QT syndrome: a simulation study. Cardiovasc Res. 1999;42:530–42. doi: 10.1016/s0008-6363(99)00035-8. [DOI] [PubMed] [Google Scholar]
- 18.Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, et al. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–8. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 19.Findlay I. Physiological modulation of inactivation in L-type Ca2+ channels: one switch. J Physiol. 2004;554:275–83. doi: 10.1113/jphysiol.2003.047902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci USA. 1990;87:753–7. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumaine R, Towbin JA, Brugada P, Vatta M, Nesterenko DV, Nesterenko VV, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–9. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 22.Brette F, Sallé L, Orchard CH. Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circ Res. 2004;95:e1–7. doi: 10.1161/01.RES.0000135547.53927.F6. [DOI] [PubMed] [Google Scholar]
- 23.Puglisi JL, Yuan W, Bassani JW, Bers DM. Ca(2+) influx through Ca(2+) channels in rabbit ventricular myocytes during action potential clamp: influence of temperature. Circ Res. 1999;85:e7–e16. doi: 10.1161/01.res.85.6.e7. [DOI] [PubMed] [Google Scholar]
- 24.Verkerk AO, Veldkamp MW, Baartscheer A, Schumacher CA, Klöpping C, van Ginneken AC, et al. Ionic mechanism of delayed afterdepolarizations in ventricular cells isolated from human end-stage failing hearts. Circulation. 2001;104:2728–33. doi: 10.1161/hc4701.099577. [DOI] [PubMed] [Google Scholar]
- 25.Harvey RD, Clark CD, Hume JR. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990;95:1077–102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.