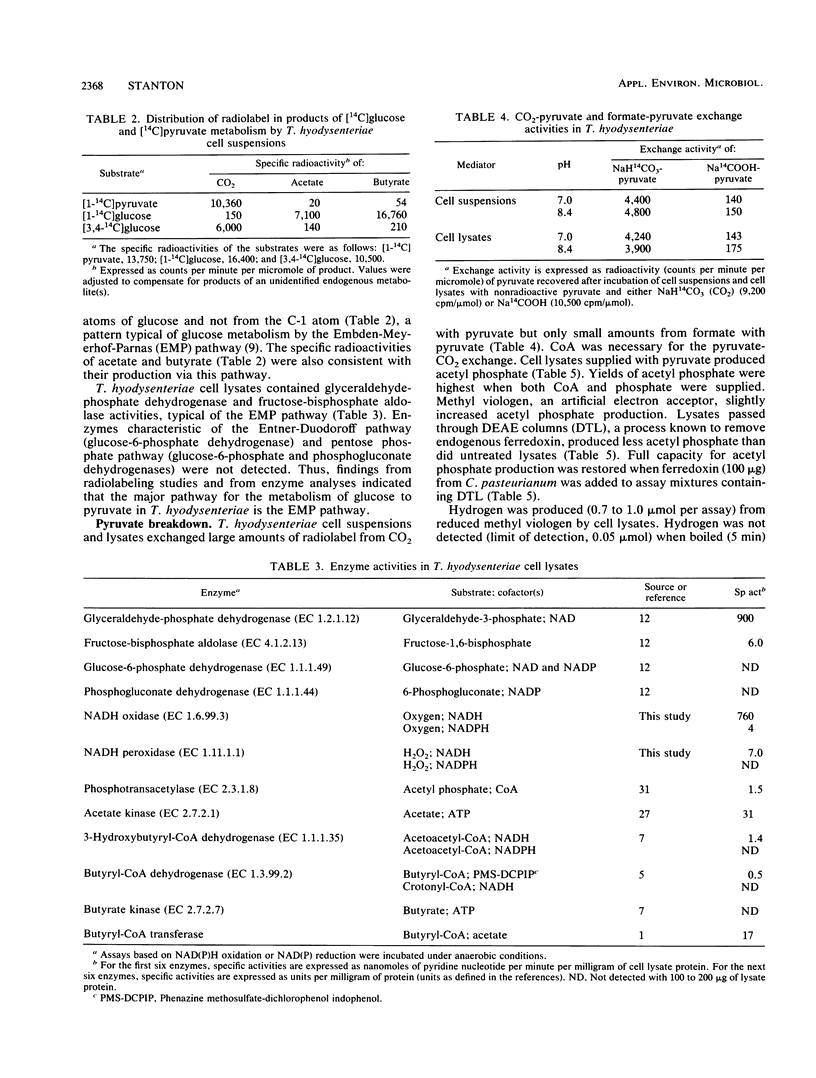
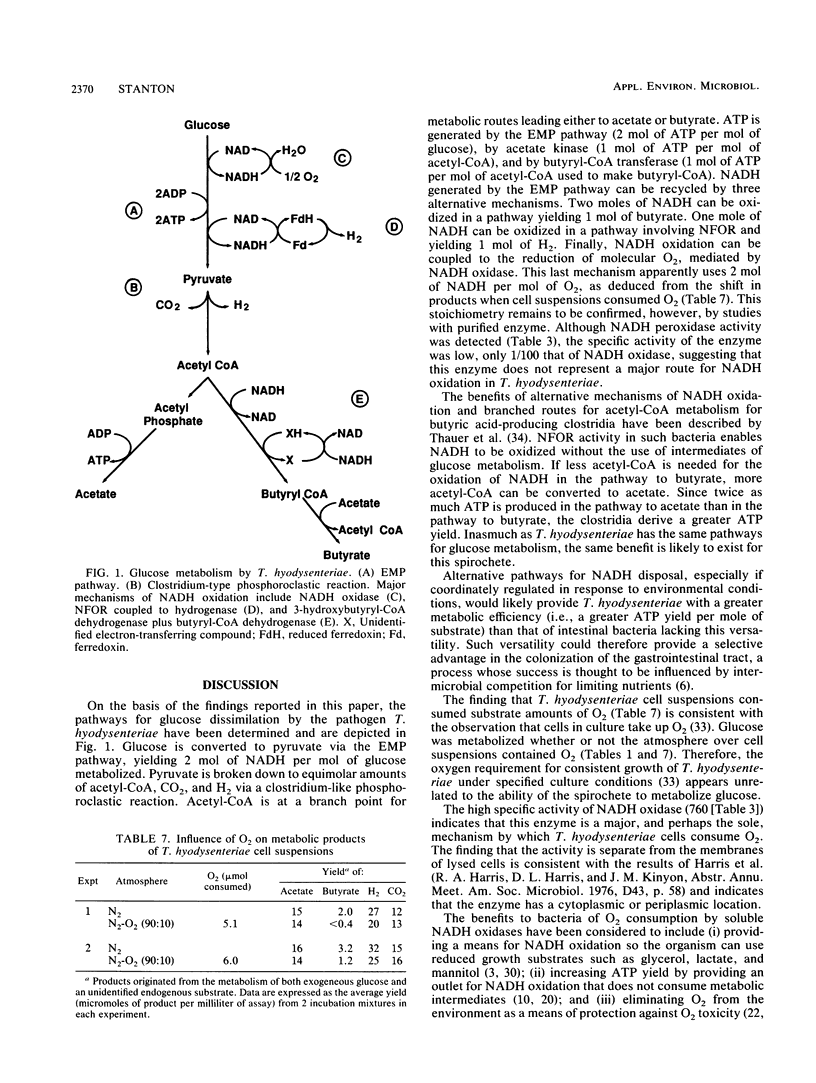
Abstract
Glucose metabolism and the mechanisms of NADH oxidation by Treponema hyodysenteriae were studied. Under an N2 atmosphere, washed cell suspensions of the spirochete consumed glucose and produced acetate, butyrate, H2, and CO2. Approximately twice as much H2 as CO2 was produced. Determinations of radioactivity in products of [14C]glucose and [14C]pyruvate metabolism and analyses of enzyme activities in cell lysates revealed that glucose was catabolized to pyruvate via the Embden-Meyerhof-Parnas pathway. The results of pyruvate exchange reactions with NaH14CO3 and Na14COOH demonstrated that pyruvate was converted to acetyl coenzyme A (acetyl-CoA), H2, and CO2 by a clostridium-type phosphoroclastic mechanism. NADH:ferredoxin oxidoreductase and hydrogenase activities were present in cell lysates and produced H2 from NADH oxidation. Phosphotransacetylase and acetate kinase catalyzed the formation of acetate from acetyl-CoA. Butyrate was formed from acetyl-CoA via a pathway that involved 3-hydroxybutyryl-coenzyme A (CoA) dehydrogenase, butyryl-CoA dehydrogenase, and butyryl-CoA transferase. T. hyodysenteriae cell suspensions generated less H2 and butyrate under 10% O2-90% N2 than under 100% N2. Cell lysates contained NADH oxidase, NADH peroxidase, and superoxide dismutase activities. These findings indicated there are three major mechanisms that T. hyodysenteriae cells use to recycle NADH generated from the Embden-Meyerhof-Parnas pathway--enzymes in the pathway from acetyl-CoA to butyrate, NADH:ferredoxin oxidoreductase, and NADH oxidase. Versatility in methods of NADH oxidation and an ability to metabolize oxygen could benefit T. hyodysenteriae cells in the colonization of tissues of the swine large bowel.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Massey V. The purification and properties of butyryl-coenzyme A dehydrogenase from Peptostreptococcus elsdenii. Biochem J. 1971 Dec;125(3):879–887. doi: 10.1042/bj1250879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Smibert R. M. Fumarate reduction and product formation by the Reiter strain of Treponema phagedenis. J Bacteriol. 1982 Dec;152(3):1049–1059. doi: 10.1128/jb.152.3.1049-1059.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glock R. D., Harris D. L., Kluge J. P. Localization of spirochetes with the structural characteristics of Treponema hyodysenteriae in the lesions of swine dysentery. Infect Immun. 1974 Jan;9(1):167–178. doi: 10.1128/iai.9.1.167-178.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant G. O., Lambert M. A., Moss C. W. Analysis of short-chain acids from anaerobic bacteria by high-performance liquid chromatography. J Clin Microbiol. 1982 Aug;16(2):355–360. doi: 10.1128/jcm.16.2.355-360.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz F., Sedewitz B., Elstner E. F. Oxygen utilization by Lactobacillus plantarum. I. Oxygen consuming reactions. Arch Microbiol. 1980 Apr;125(3):209–214. doi: 10.1007/BF00446878. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Canale-Parola E. Carbohydrate metabolism in Spirochaeta stenostrepta. J Bacteriol. 1970 Jul;103(1):216–226. doi: 10.1128/jb.103.1.216-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemcke R. M., Burrows M. R. A comparative study of spirochaetes from the porcine alimentary tract. J Hyg (Lond) 1981 Apr;86(2):173–182. doi: 10.1017/s0022172400068881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. A new procedure for assay of bacterial hydrogenases. J Bacteriol. 1956 Jan;71(1):70–80. doi: 10.1128/jb.71.1.70-80.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard B., Lariviere S., Saheb S. A. Etude comparative des caractères biochimiques de tréponèmes hémolytiques isolés du porc. Can J Microbiol. 1980 Aug;26(8):985–991. [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Ferredoxin- and nicotinamide adenine dinucleotide-dependent H 2 production from ethanol and formate in extracts of S organism isolated from "Methanobacillus omelianskii". J Bacteriol. 1972 Apr;110(1):126–132. doi: 10.1128/jb.110.1.126-132.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. L., Stöcklein W., Danzer J., Kirch P., Limbach B. Isolation and properties of an H2O-forming NADH oxidase from Streptococcus faecalis. Eur J Biochem. 1986 Apr 1;156(1):149–155. doi: 10.1111/j.1432-1033.1986.tb09560.x. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Cornell C. P. Erythrocytes as a source of essential lipids for Treponema hyodysenteriae. Infect Immun. 1987 Feb;55(2):304–308. doi: 10.1128/iai.55.2.304-308.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B., Lebo D. F. Treponema hyodysenteriae growth under various culture conditions. Vet Microbiol. 1988 Oct;18(2):177–190. doi: 10.1016/0378-1135(88)90063-6. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J Biol Chem. 1955 Aug;215(2):637–643. [PubMed] [Google Scholar]



