Abstract
Stressful events before or just after parturition alter the subsequent phenotypical response to stress in a general process termed programming. Hypoxia during the period before and during parturition, and in the postnatal period is one of the most common causes of perinatal distress, morbidity, and mortality. We have found that perinatal hypoxia (E19 to PD14) augmented the corticosterone response to stress and increased basal corticotrophin-releasing hormone mRNA levels in the parvocellular portion of the paraventricular nucleus (PVN) in 6-month old rats. There was no effect on the levels of hypothalamic parvocellular PVN vasopressin mRNA, anterior pituitary POMC or CRHR1 mRNA, or hippocampus GR mRNA. We conclude that hypoxia spanning the period just before and for several weeks after parturition programs the hypothalamic-pituitary-adrenal axis to hyper-respond to acute stress in adulthood probably from drive from the parvocellular CRH neurons.
Keywords: Hypoxia, Perinatal, Programming, Corticosterone, Corticotropin-releasing hormone
Introduction
Hypoxia during the period before and during parturition, and in the postnatal period is one of the most common causes of perinatal distress, morbidity, and mortality (1–4). We have extensively characterized the short-term metabolic, endocrine, and growth effects of hypoxia in the pre- and neonatal period of the rat (5–13). It has been increasingly clear that perinatal events have significant and sometimes dramatic effects on the physiological phenotype of the adult, a phenomenon termed fetal or neonatal “programming” (14–23).
There have been a few studies demonstrating long-lasting effects of pre- and post-natal hypoxia on cerebral function (24), pulmonary hemodynamics (25), behavior responses to stress (26,27), chemoreflexes (38), and sympathoadrenal function (29). Some components of the adult hypothalamic-pituitary-adrenal (HPA) axis are also affected by prolonged fetal or acute post-natal hypoxia, although the previous studies are not consistent in that some show augmentation and some inhibition of the response to stress depending on when during development, for how long, and to what degree the hypoxia was applied (30–32). Furthermore, these studies have not evaluated the central neural mechanisms of long-term effects of perinatal hypoxia on the HPA axis of the adult. Complicating matters is that perinatal hypoxia decreases food intake and growth and increases endogenous corticosterone and insulin levels (5–7), which may also independently program subsequent changes in the adult phenotype (17,21,33).
The purpose of the present study was to evaluate the effect of pre- and post-natal (i.e. perinatal) hypoxia on the corticosterone responses to stress and correlate them with central and pituitary indices of HPA axis control in adult rats. This report will focus on the effect of perinatal hypoxia on the subsequent levels of hypothalamic paraventricular parvocellular corticotrophin-releasing hormone (CRH) and vasopressin mRNA, hippocampal glucocorticoid receptor mRNA, anterior pituitary POMC and CRH receptor-1 (CRHR1) mRNA, and on the adrenal corticosterone response to acute stress in adult rats.
Materials and Methods
All animal procedures were approved by the Aurora Health Care Institutional Animal Care and Use Committee. Animals were housed with a 6AM on – 6PM off light cycle. Thirty-three 6 month-old male rats from 13 litters were studied. (2–3 males per litter were studied.) Pregnant dams were obtained at 14 days gestation. Perinatal hypoxia was induced by placing pregnant dams in an environmental chamber vented with 12% O2 at E19, allowing parturition to occur in the hypoxic environment, and then keeping the pups and dams in the hypoxia chamber until 14 days of age (post-natal day (PD) 14)(34). This level of inspired oxygen results in an arterial PO2 in adults of about 50–55 mmHg (35-37). Normoxic controls were exposed to room air (21% O2) from E19 through PD14.
Weaning was at PD21, at which time male and female offspring were separated. Only male rats were studied as adults. A subset of rats was weighed at PD14, 20, 28, 33, and then not again until 5 and 6 months of age.
At 6 months of age, rats previously exposed to perinatal normoxia (controls; N=15 from 6 litters) or hypoxia (N=18 from 7 litters) were accustomed to handling for one week before being studied, as described previously (38). Restraint stress was applied for 60 min, with repeated blood for corticosterone obtained from each rat by repeated tail-nick before (0 min), at 30 and 60 min of restraint, and at 90, and 150 min after the start of restraint stress (30 and 90 min after return to home cages) as described previously (38). At least one week later, a subset of adult rats (from 7 litters) exposed to perinatal hypoxia (N = 5) and normoxic controls (N= 6) were decapitated between 8–10 am without restraint stress. Anterior pituitaries and brains were dissected and frozen for subsequent analysis as described previously (8). Another subset of adult rats (N=13 [7 normoxic/6 hypoxic] from 6 litters) was sampled by repeated tail-nicks at 8 AM and 5 PM for the assessment of the diurnal rhythm in corticosterone.
Plasma corticosterone was measured by radioimmunoassay (9,10). In situ hybridization histochemistry (ISHH) was used to assess hypothalamic paraventricular (PVN) CRH and AVP mRNA expression (8) and hippocampal glucocorticoid receptor mRNA (36). All ISHH data were analyzed by digitizing the X-ray images, with optical densities calculated using NIH Image software (courtesy W. Rasband, NIH) with a program that analyzed only signals exceeding 3.5x background. Data were expressed as mean gray values. (We did not have sufficient brain sections to analyze hippocampal mineralocorticoid receptor mRNA). Ectopic AVP-expressing magnocellular neurons in the parvocellular division of the PVN were eliminated from analyses using a thresholding technique. This was done in NIH image software using an additional program that eliminated any signal above 5x the mean intensity signal from the selected area (represented by ectopic magnocellular neurons).
Anterior pituitary POMC mRNA expression was assessed by Northern analysis as described previously (8). Anterior pituitary CRHR1 mRNA levels were assessed by similar Northern blotting techniques using probes transcribed from a 461 bp rat CRHR1 cDNA clone kindly provided by Neurocrine Biosciences (San Diego, CA). Data from Northern analyses were normalized to 28S mRNA levels.
Data were analyzed by t-test or 2-factor analysis of variance repeated on one factor (time of restraint stress), followed by Newman-Keuls multiple range test (Sigmastat 2.03). The integrated corticosterone response to stress was calculated as the area under the curve above baseline by the trapezoidal rule. Data are presented as mean ± standard error, with P<0.05 considered significant.
Results
Rats exposed to perinatal (E19-PD14) hypoxia had decreased body weight gain (Figure 1). Although their weights were still lower at 5 months of age, adult male rats exposed to perinatal hypoxia gained weight from 5 to 6 months of age in parallel with normoxic controls. Adrenal mass normalized to body weight (gm/gm) in adults exposed to perinatal hypoxia (0.17±0.02) was not statistically different from normoxic controls (0.14±0.01). A significant diurnal variation in plasma corticosterone was present in adult rats exposed to perinatal normoxia, with morning (24±14 ng/ml; N=6) being lower than evening levels (125±32 ng/ml; N=7; P<0.001). In adult rats exposed to perinatal hypoxia, the morning (30±9 ng/ml; N=6) and evening (114±21 ng/ml; N=6) unstressed plasma corticosterone levels were not different from normoxic controls.
Figure 1.
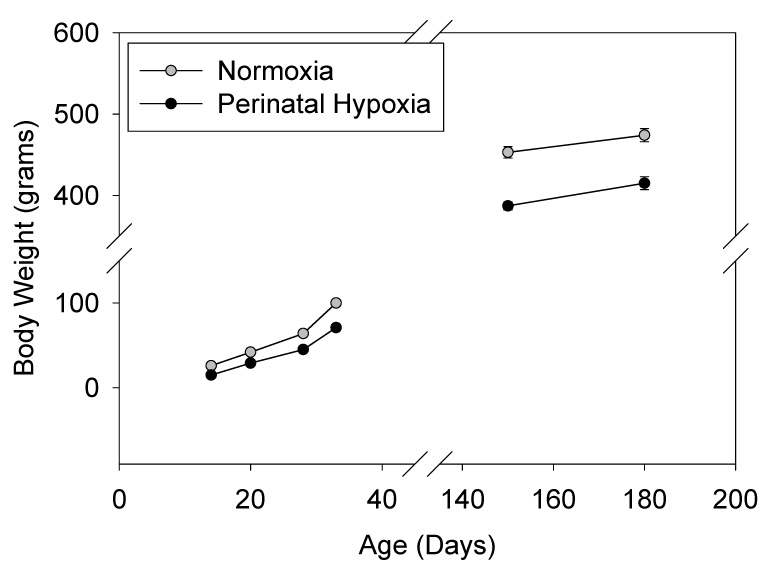
Body weight at post-natal day (PD) 14–33, and at 5 and 6 months of age in rats exposed to perinatal normoxia vs. hypoxia from E19 (in utero) to PD14. N=7–8 per treatment group from 4 litters per treatment group. SEMs are smaller than the symbol size. Perinatal hypoxia body weights were significantly less than normoxic controls at each time point (P<0.001).
Figure 2 shows the corticosterone response to restraint in adult rats exposed to perinatal normoxia (controls; N=15) or hypoxia (from E19 to PD14; N=18). Basal corticosterone levels were not different between groups (Figure 2 left panel). However, plasma corticosterone was significantly higher at all times measured during and after restraint in rats exposed to perinatal hypoxia. This enhanced corticosterone response to restraint was further demonstrated by the augmented integrated corticosterone response to restraint shown on the right panel of Figure 2.
Figure 2.
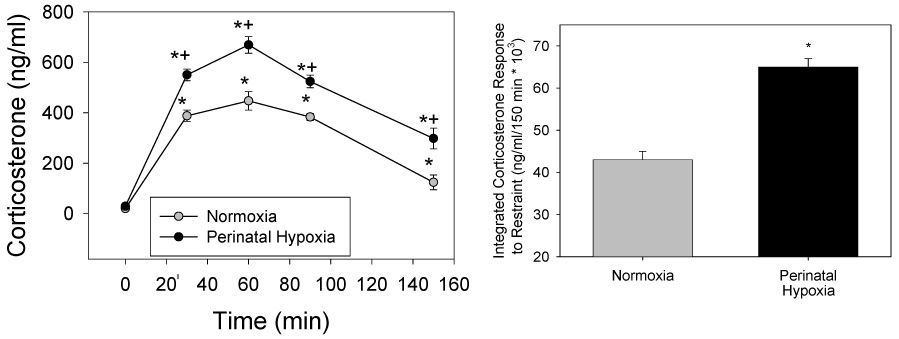
Left: Corticosterone response to restraint applied from 0–60 min in rats at 5–6 months of age. Rats were exposed to perinatal hypoxia (E19-PD14; N=18 from 7 litters) or normoxia (controls; N=15 from 6 litters). *different from 0 min; +different from normoxic control. Right: Integrated (area under curve minus baseline corticosterone) corticosterone response to restraint. *different from normoxia. (P<0.001)
Figure 3 shows the analysis of basal CRH mRNA levels in the parvocellular paraventricular nucleus (PVN) of the hypothalamus in adult rats exposed to perinatal normoxia (N=6) or hypoxia (N=5). There was a significant increase in parvocellular CRH mRNA in adult rats exposed to perinatal hypoxia compared to normoxic controls. There was no effect on parvocellular AVP levels in the PVN in adult rats exposed to perinatal normoxia vs. hypoxia (48.9±1.1 vs. 51.5±2.7 mean gray area; N=5–6 per mean). There was also no difference between adult rats exposed to perinatal normoxia vs. hypoxia in POMC mRNA levels (1.1±0.2 vs. 1.2±0.3 normalized to 28S; N=4–6 per mean) or CRHR1 mRNA levels (8.0±0.6 vs. 10.8±1.7 normalized to 28S; N=6–8 per mean) in the anterior pituitary of adult rats. Finally, there were no differences in hippocampal glucocorticoid receptor mRNA levels (N=4–6 per mean) in adult rats exposed to perinatal normoxia vs.hypoxia in the CA1 (24±3 vs. 26±3 mean gray area), CA3 (9.6±2.4 vs. 10.8±1.3 mean gray area), or dentate gyrus (27.6±1.2 vs. 27.8±2.4 mean gray area).
Figure 3.
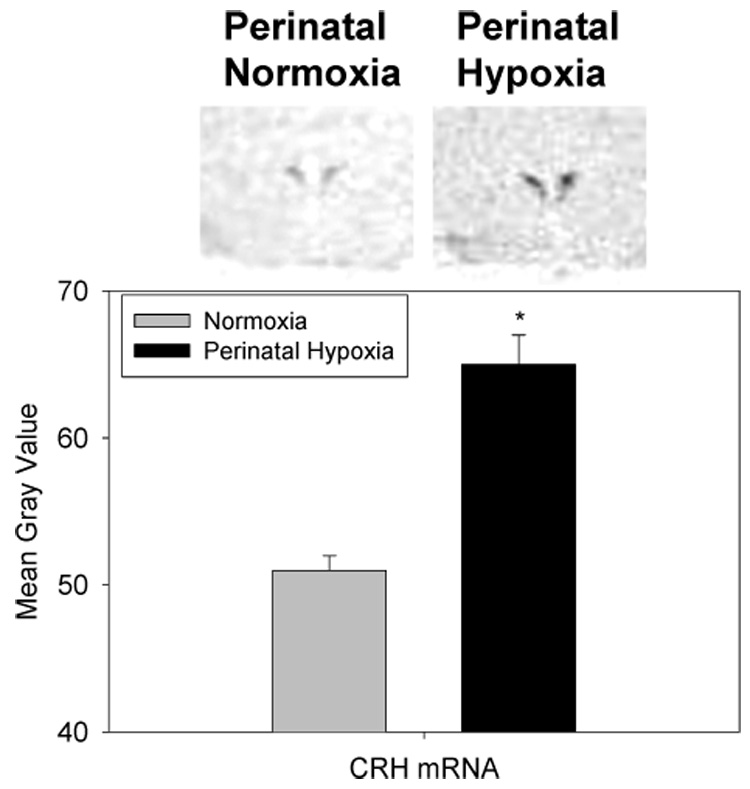
Parvocellular CRH in the paraventricular nucleus of adult rats exposed to normoxia or perinatal hypoxia (E19-PD14) by in situ hybridization histochemistry (top). The image above is an example of a CRH mRNA histochemistry image from digitized images from x-ray films. N=5–6 brains per mean/ from 4 litters per mean. *different from normoxia (P<0.001).
Discussion
This study demonstrated that exposure to perinatal hypoxia from the last two days of gestation to 14 days after parturition resulted in an augmented corticosterone response to restraint in adult rats compared to those exposed to a control, normoxic environment in the perinatal period. The enhanced corticosterone response to an acute stressor occurred even though basal corticosterone levels were not elevated in rats exposed to perinatal hypoxia. CRH mRNA levels in the parvocellular paraventricular nucleus of the hypothalamus were increased in adult rats exposed to perinatal hypoxia compared to normoxic controls. There was no effect of perinatal hypoxia on anterior pituitary POMC or CRHR1, parvocellular hypothalamic PVN AVP, or hippocampal glucocorticoid mRNA levels.
Hypoxia is one of the more common neonatal stressors and is known to have dramatic acute effects on virtually every organ and physiological function studied (1–13). Exposure to prenatal hypoxia for most of gestation (E5 to E20) has also been shown to result in an augmented corticosterone to stress in the adult, whereas brief exposures to post-natal hypoxia have not shown a similar effect (26,31,32). This is consistent with the current study, which found an effect of prolonged hypoxia extending from the 2 days just before parturition to 14 days of age. It may be, then, that the programming of the adult HPA axis by hypoxia in the perinatal period requires a relatively long exposure to low oxygen.
This is the first study we are aware of that suggests a possible mechanism for the programming of adult HPA axis function by hypoxia in the perinatal period. The data suggest that augmented hypothalamic parvocellular CRH levels may be the cause of enhanced adult corticosterone responses to stress after perinatal hypoxia. The data analysis using digitized x-ray films does not permit the determination of an effect due to increased number of CRH-expressing cells. The lack of an effect of perinatal hypoxia on subsequent adult levels of anterior pituitary POMC or CRHR1 mRNA, or of hippocampal GR, suggests that the augmentation of the corticosterone response to restraint stress is probably caused by drive from the parvocellular CRH neurons rather than by a change in feedback sensitivity or corticotroph responsiveness.
There are other factors that may influence the interpretation of the data. We have previously demonstrated (and confirmed in this study) that post-natal exposure to hypoxia results in a decrease in pup weight gain (40). Although weight gain does recover, the current study demonstrated that there is no catch-up growth. Despite parallel rates of weight gain in adulthood, actual weight remains lower in rats previously exposed to hypoxia. This effect does not seem to be mediated by changes in thyroid hormone, growth hormone, insulin-like growth factor 1, or parathyroid hormone (5–7,9,12). Since early-life undernutrition has been shown to program the subsequent HPA axis phenotype (41), it is possible that lower body weight gain in the rat pups exposed to hypoxia was a significant factor in the augmentation of the corticosterone response to restraint that we observed. Finally, it is possible that changes in maternal factors caused by hypoxia such as changes in food intake or behavior, may have contributed to the effect observed in the offspring (18,21,22,41). It is also important not to over-interpret the mRNA data, since it is always possible that they do not accurately reflect CRH protein synthesis or secretion.
We have also previously demonstrated an increase in basal corticosterone during neonatal hypoxia (10,13). It is important to note that this is quite a different phenomenon than what we have discovered in the present study. The acute, neonatal increase in basal corticosterone during hypoxia is (a) not mediated by the hypothalamus, (b) normalized by chemical sympathectomy, and (c) not sustained, in that the increase in corticosterone normalizes soon after return of the neonate to normoxia (8–10). In fact, the response to stress or CRH is attenuated during neonatal hypoxia due to glucocorticoid negative feedback (8,13). However, although driven by a different mechanism, it is possible that the increase corticosterone during neonatal hypoxia could have “programmed” the brain to alter the regulation of hypothalamic CRH mRNA and adrenocortical responses to stress in the adult. This does not appear to be a likely mechanism since increases in neonatal glucocorticoid exposure appears to attenuate subsequent HPA axis response to stress (42–45) despite a downregulation of central glucocorticoid receptor expression (46).
Finally, increases in insulin, probably due to corticosterone-mediated decreases in insulin sensitivity during neonatal hypoxia (6), may have also contributed to changes in the adult HPA axis phenotype, as has been demonstrated for metabolic and cardiovascular control (47). Therefore, it is possible that the “programming” induced by hypoxia may have been modified by decreases in food intake and weight gain, and changes in maternal behavior, corticosterone and/or insulin levels.
What might be the mechanism by which the exposure to hypoxia in the perinatal period leads to an increase in CRH mRNA levels and augmented corticosterone responses to stress months after the exposure, despite normal basal corticosterone levels in the morning and evening? It is possible that the control of the CRH gene promoter is altered by epigenetic phenomena such as changes in DNA methylation, histone function, or some other mechanism (48). Even though CRH gene expression is increased, CRH release from the parvocellular paraventricular neurons might be relatively normal under basal conditions but could be augmented when stimulated by stress, leading ultimately to greater stress-induced corticosterone secretion.
The possibility of a dissociation between gene expression and subsequent physiological effects has been suggested previously (15,17,22,49,50). There are two quite pertinent examples in adult rats of increased CRH mRNA expression and increased HPA axis responsiveness to stress despite completely normal basal, unstressed plasma ACTH and corticosterone. Of particular interest is that neonatal maternal separation results in subsequent increases in PVN CRH mRNA, and ACTH and corticosterone responses to airpuff startle, but normal basal plasma ACTH and corticosterone (51). This raises the possibility mentioned earlier that hypoxia-induced changes in maternal behavior could have been a component of the response we report here. A difference is that neonatal maternal separation altered central GR mRNA (51), whereas we did not find such an effect. Repeated stress applied to adult rats also results in increased CRH mRNA levels in the PVN, augmented HPA axis responses to a novel stressor, and normal, basal plasma ACTH and corticosterone (52,53). In this case, as opposed to our study, there is decreased GR expression in the hippocampus suggesting a negative feedback mechanism. Therefore, the phenomenon of the association of increased central drive from the CRH neuron with increased stress-induced, but not basal ACTH and corticosterone, is well described. Some of the putative mechanisms of this phenomenon suggest that perinatal hypoxia may have some unique characteristics not necessarily attributable to changes in maternal care and/or the stress itself. It is also possible that the observed effects are a reflection of hypoxia-induced programming within regulatory afferents of the stress-integrative CRH neurons, which may undergo a shift toward enhanced excitation with exposure to acute stressful stimuli in the adult.
Perinatal hypoxia is a common, complex early-life stress. This raises the possibility that subsequent HPA function and, in particular, the response to acute stressors, could be altered in adolescents and adults and may require lifelong vigilance in following patients who experience a prolonged hypoxic episode in the perinatal period (54,55).
Acknowledgements
Supported by NIH grant DK54685 to HR, Albany Medical College intramural funds to LJ, and MH56577 to WEC. The authors thank Eric Bruder, Peter Homar, Barbara Jankowski, Rebecca Rokow-Kittell and Lisa Gallatin for their expert assistance.
References
- 1.Frankel L, Stevenson DK. Metabolic emergencies of the newborn: hypoxemia and hypoglycemia. Comprehensive Therapy. 1987;13:14–19. [PubMed] [Google Scholar]
- 2.Friedman AH, Fahey JT. The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Seminars in Perinatology. 1993;17:106–121. [PubMed] [Google Scholar]
- 3.Gronget JF. Metabolic consequences of induced hypoxia in newborn lambs. Annales de Recherches Veterinaires. 1984;15:17–28. [PubMed] [Google Scholar]
- 4.Zayour D, Azar ST, Azar N, Nasser M, Obeid M, Mroueh S, Dbaibo GS, Bitar FF. Endocrine changes in a rat model of chronic hypoxia mimicking cyanotic heart disease. Endocrine Research. 2003;29:191–200. doi: 10.1081/erc-120022301. [DOI] [PubMed] [Google Scholar]
- 5.Raff H, Bruder ED, Jankowski BM. The effect of hypoxia on plasma leptin and insulin in newborn and juvenile rats. Endocrine. 1999;11:37–39. doi: 10.1385/ENDO:11:1:37. [DOI] [PubMed] [Google Scholar]
- 6.Raff H, Bruder ED, Jankowski BJ, Colman RJ. Effect of neonatal hypoxia on leptin, insulin, growth hormone and body composition in the rat. Horm Metab Res. 2001;33:151–155. doi: 10.1055/s-2001-14929. [DOI] [PubMed] [Google Scholar]
- 7.Raff H, Bruder ED, Jankowski BM, Oaks MK, Colman RJ. Growth hormone therapy during neonatal hypoxia in rats: body composition, bone mineral density, and insulin-like growth factor-1 expression. Endocrine. 2001;16:139–143. doi: 10.1385/ENDO:16:2:139. [DOI] [PubMed] [Google Scholar]
- 8.Raff H, Jacobson L, Cullinan WE. Elevated corticosterone and inhibition of ACTH responses to CRH and ether in the neonatal rat: effect of hypoxia from birth. Am J Physiol Regulat Integrat Compar Physiol. 2003;285:R1224–R1230. doi: 10.1152/ajpregu.00259.2003. [DOI] [PubMed] [Google Scholar]
- 9.Raff H, Jankowski BM, Bruder ED, Engeland WC, Oaks MK. The effect of hypoxia from birth on the regulation of aldosterone in the 7-day-old rat: plasma hormones, steroidogenesis in vitro, and steroidogenic enzyme messenger ribonucleic acid. Endocrinology. 1999;140:3147–3153. doi: 10.1210/endo.140.7.6794. [DOI] [PubMed] [Google Scholar]
- 10.Raff H, Lee JJ, Widmaier EP, Oaks MK, Engeland WC. Basal and adrenocorticotropin-stimulated corticosterone in the neonatal rat exposed to hypoxia from birth: modulation by chemical sympathectomy. Endocrinology. 2004;145:79–86. doi: 10.1210/en.2003-1130. [DOI] [PubMed] [Google Scholar]
- 11.Raff H, Bruder ED, Jankowski BM, Engeland WC. The effect of fetal hypoxia on adrenocortical function in the 7-day old rat. Endocrine. 2000;13:111–116. doi: 10.1385/ENDO:13:1:111. [DOI] [PubMed] [Google Scholar]
- 12.Raff H. Effect of hypoxia on parathyroid hormone in lactating and neonatal rats: interaction with halothane. Endocrine. 2002;17:157–160. doi: 10.1385/ENDO:17:3:157. [DOI] [PubMed] [Google Scholar]
- 13.Raff H, Jacobson L. Glucocorticoid feedback control of corticotropin (ACTH) in the hypoxic neonatal rat. J. Endocrinol. 2007;192:453–458. doi: 10.1677/JOE-06-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews MH, Matthews SG. Programming of the hypothalamo-pituitary-adrenal axis: serotonergic involvement. Stress. 2004;7:15–27. doi: 10.1080/10253890310001650277. [DOI] [PubMed] [Google Scholar]
- 15.Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J. Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- 16.Ellis S, Mouihate A, Pittman QJ. Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics. J Physiol. 2006;571:695–701. doi: 10.1113/jphysiol.2005.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hales CN, Ozanne SE. For Debate: Fetal and early postnatal growth restrictions lead to diabetes, the metabolic syndrome and renal failure. Diabetologia. 2003;46:1013–1019. doi: 10.1007/s00125-003-1131-7. [DOI] [PubMed] [Google Scholar]
- 18.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson C, Jennische E, Ho HP, Eriksson E, Bjorntorp P, Holmang A. Postnatal endotoxin exposure results in increased insulin sensitivity and altered activity of neuroendocrine axes in adult female rats. European Journal of Endocrinology. 2002;146:251–260. doi: 10.1530/eje.0.1460251. [DOI] [PubMed] [Google Scholar]
- 20.Panagiotaropoulos T, Papaioannou A, Pondiki S, Prokopiou A, Stylianopoulou F, Gerozissis K. Effect of neonatal handling and sex on basal and chronic stress-induced corticosterone and leptin secretion. Neuroendocrinology. 2004;79:109–118. doi: 10.1159/000076633. [DOI] [PubMed] [Google Scholar]
- 21.Suchecki D, Tufik S. Long-term effects of maternal deprivation on the corticosterone response to stress in rats. Am J Physiol Regulatory Integrative Comp Physiol. 1997;273:R1332–R1338. doi: 10.1152/ajpregu.1997.273.4.R1332. [DOI] [PubMed] [Google Scholar]
- 22.Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 23.Young JB. Effects of neonatal handling on sympathoadrenal activity and body composition in adult male rats. Am J Physiol Regulatory Integrative Comp Physiol. 2000;279:R1745–R1752. doi: 10.1152/ajpregu.2000.279.5.R1745. [DOI] [PubMed] [Google Scholar]
- 24.Vannucci RC, Rossini A, Towlighi J, Vannucci SJ. Measuring the accentuation of the brain damage that arises from perinatal cerebral hypoxia-ischemia. Biol Neonate. 1997;72:187–191. doi: 10.1159/000244483. [DOI] [PubMed] [Google Scholar]
- 25.Hampl V, Bibova J, Herget Perinatal history of hypoxia leads to lower vascular pressures and hyporeactivity to angiotensin II in isolated lungs of adult rats. Physiol Res. 2000;49:567–575. [PubMed] [Google Scholar]
- 26.Boksa P, Wilson D, Rochford J. Responses to stress and novelty in adult rats born vaginally, by cesarean section, or by cesarean section with acute anoxia. Biol Neonate. 1998;74:48–50. doi: 10.1159/000014010. [DOI] [PubMed] [Google Scholar]
- 27.El-Khodor BG, Boksa P. Transient birth hypoxia increases behavioral responses to repeated stress in the adult rat. Behav Brain Res. 2000;107:171–175. doi: 10.1016/s0166-4328(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 28.Peyronnet J, Roux JC, Geloen A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y. Prenatal hypoxia impairs postnatal development of neural and functional chemoafferent pathway in rat. J Physiol. 2000;524:525–537. doi: 10.1111/j.1469-7793.2000.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soulier V, Peyronnet J, Pequignot JM, Cottet-Emard JM, Lagercrantz H, Dalmaz Y. Long-term impairment in the neurochemical activity of the sympathoadrenal system after neonatal hypoxia in the rat. Pediatr Res. 1997;42:30–38. doi: 10.1203/00006450-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Boksa P, Krishnamurth A, Sharma S. Hippocampal and hypothalamic type I corticosteroid receptor affinities are reduced in adult rats born by a caesarean procedure with or without an added period of anoxia. Neuroendocrinology. 1996;64:25–34. doi: 10.1159/000127094. [DOI] [PubMed] [Google Scholar]
- 31.Joseph V, Mamet J, Lee F, Dalmaz Y, Van Reeth O. Prenatal hypoxia impairs circadian synchronization and response of the biological clock to light in adult rats. J. Physiol. 2002;543:387–395. doi: 10.1113/jphysiol.2002.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyakas C, Buwalda B, Markel E, Korte SM, Luiten PGM. Life-spanning behavioural and adrenal dysfunction induced by prenatal hypoxia in the rat is prevented by the calcium antagonist nimodipine. Eur J Neurosci. 1994;6:746–753. doi: 10.1111/j.1460-9568.1994.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 33.Dugovic C, Maccari S, Weibel L, Turek FS, Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. J Neurosci. 1999;19:8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas T, Marshall JM. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. J Physiol. 1995;487:513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raff H, Chadwick CJ. Aldosterone responses to ACTH during hypoxia in conscious rats. Clin Exp Pharmacol Physiol. 1986;13:827–830. doi: 10.1111/j.1440-1681.1986.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 36.Raff H, Roarty TP Renin. ACTH, and aldosterone during acute hypercapnia and hypoxia in conscious rats. AmJ Physiol Regul Integr Comp Physiol. 1988;254:R431–R435. doi: 10.1152/ajpregu.1988.254.3.R431. [DOI] [PubMed] [Google Scholar]
- 37.Raff H, Sandri RB, Segerson TP Renin. ACTH, and adrenocortical function during hypoxia and hemorrhage in conscious rats. AmJ Physiol Regul Integr Comp Physiol. 1986;250:R240–R244. doi: 10.1152/ajpregu.1986.250.2.R240. [DOI] [PubMed] [Google Scholar]
- 38.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern of time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1994;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 39.Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Molecular Endocrinology. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- 40.Bruder ED, Lee PC, Raff H. 2004 Metabolic consequences of hypoxia from birth and dexamethasone treatment in the neonatal rat: comprehensive hepatic lipid and fatty acid profiling. Endocrinology. 2004;145:5364–5372. doi: 10.1210/en.2004-0582. [DOI] [PubMed] [Google Scholar]
- 41.Leonhardt M, Lesage J, Dufourny L, Dickes-Coopman A, Montel V, Doupoy J-P. Perinatal maternal food restriction induces alterations in hypothalamo-pituitary-adrenal axis activity and in plasma corticosterone-binding globulin capacity of weaning rat pups. Neuroendocrinology. 2002;75:45–54. doi: 10.1159/000048220. [DOI] [PubMed] [Google Scholar]
- 42.Flagel SB, Vasquez DM, Watson SJ, Neal CR. Effects of tapering neonatal dexamethasone on rat growth, neurodevelopment, and stress response. Am J Physiol Integrat Comp Physiol. 2002;282:R55–R63. doi: 10.1152/ajpregu.2002.282.1.R55. [DOI] [PubMed] [Google Scholar]
- 43.Theogaraj E, John CD, Christian HC, Morris JF, Smith SF, Buckingham JC. Perinatal glucocorticoid treatment produces molecular, functional, and morphological changes in the anterior pituitary gland of the adult male rat. Endocrinology. 2005;146:4804–4813. doi: 10.1210/en.2005-0500. [DOI] [PubMed] [Google Scholar]
- 44.Glover V, Miles R, Matta S, Modi N, Stevenson J. Glucocorticoid exposure in preterm babies predicts saliva cortisol response to immunization at 4 months. Pediatric Research. 2005;58:1233–1237. doi: 10.1203/01.pdr.0000185132.38209.73. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson C, Jennische E, Ho HP, Eriksson E, Bjorntorp P, Holmang A. Increased insulin sensitivity and decreased body weight in female rats after postnatal corticosterone exposure. European Journal of Endocrinology. 2002;146:847–854. doi: 10.1530/eje.0.1460847. [DOI] [PubMed] [Google Scholar]
- 46.Felszeghy Y, Gaspar E, Nyakas C. Long-term selective down-regulation of brain glucocorticoid receptors after neonatal dexamethasone treatment in rats. J. Neuroendocrinol. 1996;8:493–499. doi: 10.1046/j.1365-2826.1996.04822.x. [DOI] [PubMed] [Google Scholar]
- 47.Dorner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity, and enhanced cardiovascular risk in later life. Horm Metab Res. 1994;26:213–221. doi: 10.1055/s-2007-1001668. [DOI] [PubMed] [Google Scholar]
- 48.Newell-Price J, Clark AJL, King P. DNA methylation and silencing of gene expression. Trends Endocrinol Metab. 2000;11:142–148. doi: 10.1016/s1043-2760(00)00248-4. [DOI] [PubMed] [Google Scholar]
- 49.Pryce CR, Ruedi-Bettschen D, Dettling AC, Feldon J. Early life stress: long-term physiological impact in rodents and primates. News Physiol Sci. 2002;17:150–155. doi: 10.1152/nips.01367.2001. [DOI] [PubMed] [Google Scholar]
- 50.Rakyan VK, Presi J, Morgan HD, Whitelaw E. The marks, mechanisms and memory of epigenetic states in mammals. Biochem J. 2001;356:1–10. doi: 10.1042/0264-6021:3560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–289. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 52.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress-paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 53.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 54.Prancota J. Possible pathomechanisms of sudden infant death syndrome: key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am J Therapeut. 2004;11:517–546. doi: 10.1097/01.mjt.0000140648.30948.bd. [DOI] [PubMed] [Google Scholar]
- 55.Raman L, Georgieff MK, Rao R. The role of chronic hypoxia in the development of neurocognitive abnormalities in preterm infants with bronchopulmonary displasia. Develomental Sci. 2006;9:359–367. doi: 10.1111/j.1467-7687.2006.00500.x. [DOI] [PubMed] [Google Scholar]


