Abstract
The effect of interval, direction and rate of strain on mechanotransduction in neonatal rat cardiomyocytes is determined for focal adhesion kinase (Y397pFAK), extracellular signal-regulated kinase ERK1/2 (Thr183/Tyr185) and paxillin (pY31) and phosphorylation time courses to 10% strain assessed. Cells are non-responsive at 5-minutes but recover at 15 minutes (p<0.03) with FAK nuclear translocation by 30-minutes. Cyclic biaxial strain increased phosphorylation from slower to faster rates (p<0.05). Uniaxial strain to groove-aligned myocytes increased FAK and ERK1/2 phosphorylation transversely more than longitudinally (p<0.05). Mechanotransduction may have a refractory period of 5-minutes and differentiate directions and rates of strain.
Keywords: Cardiomyocytes, Force direction, Mechanotransduction, Focal adhesion kinase, Refractory period, Nucleo-cytoplasmic transport
Introduction
A ventricular myocyte experiences changes in length and load with increased systolic wall stress due to pressure, or diastolic wall stress due to volume overload. These are transduced into biochemical signals that change the rate of protein synthesis, cell morphology, protein localization, phosphorylation and gene expression. The chemical agents that trigger signaling candidates are frequently studied but the equally important physical modes are less studied. Mechanical variables include the magnitude of load or strain, material stiffness, flow, shear stress and compression [1,2]. The direction of force affects responses of aligned tissue or cell culture [3-7].
Mechanotransduction begins at the focal adhesion complex as cells sense physical forces and transduce them to activate signaling cascades by phosphorylation of FAK, ERK, paxillin and others [8]. The nature and timing of the mechanical stimuli greatly affects the ability of the myocyte to detect changes. Here, the interval between sudden strains is changed to test for presence of a refractory period by diminution of FAK and ERK phosphorylation. Changing the strain rate and strain direction simulates transverse stress in pressure overload and longitudinal strain in volume overload to test for rate and anisotropy of responses.
Materials and Methods
Cell Culture
Animal experiments were performed according to Institutional Animal Care and Use Committee and NIH guidelines. Neonatal rat ventricular myocytes (NRVM) were isolated from the hearts of 1-2 day-old Sprague-Dawley rats (Harlan, Indianapolis, IN) and plated on fibronectin-coated (12.5 μL/mL) silicone membranes (200, 000 cells/cm2). Standard methods were used for static [9], strain vector and rate studies [10]. Sigma antibiotic solution (5 μL/mL) was sometimes excluded for 48 hours.
Mechanical strain
Static strain
Silicone membranes (Specialty Manufacturing Inc, Saginaw, MI) were mounted on strain devices [3] for 10% strain application [9] of either 5 or 15 minutes followed by a second strain of the same duration.
Cyclic strain
Mechanical deformation was varied for rate and vector (Model FX-4000™, Flexercell International, McKeesport, PA). NRVM were strained 10% biaxially (BioFlex®) for 20 min in non-serum media at varied frequency to modulate the rate of strain expressed in %strain/time (% s-1).
Strain Vector
Microfabricated grooved substrata (5μm deep, 10μm valley and ridge (Figure 1) [11] were made in uniaxial dishes (Uniflex™) by pressing the parylene mold against unpolymerized silicone (Dupont, Phoenix, AZ). NRVM were plated on grooves set perpendicular to the uniaxial stretch axis for transverse and parallel for the longitudinal 10% strain for 20 minutes at strain at about 1Hz.
Figure 1. Aligned NRVM and nuclear translocation.
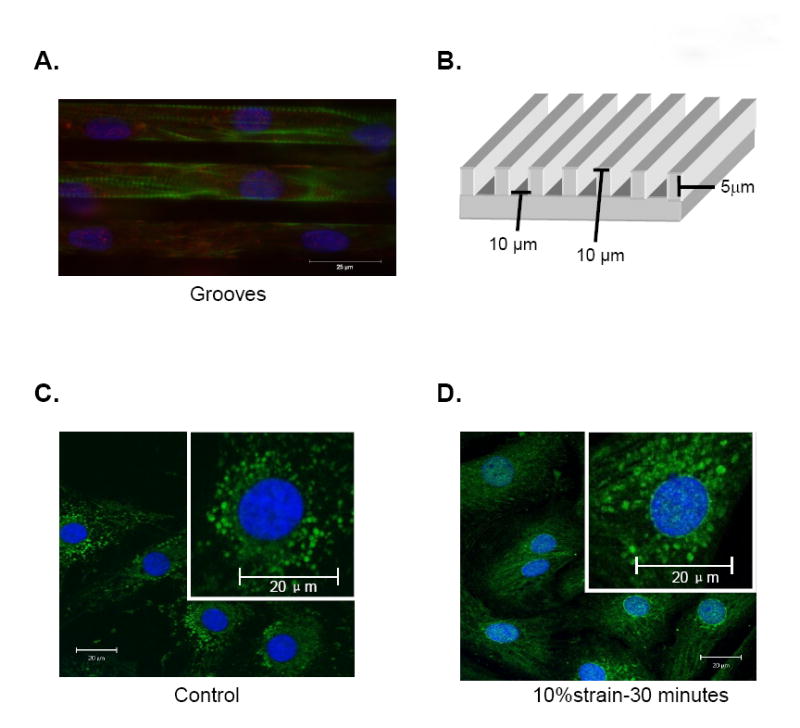
(A) NRVM on microtextured grooves. FAKpY397 (red), actin (green), nucleus (DAPI, blue). (B) Substrata diagram with dimensions. (C, D) NRVM on flat membrane. Total FAK (green).
Western Blots and Immunochemistry
Proteins were probed with antibodies: FAK (BD transduction, Lexington, KY); pY397 FAK (Biosource, Camarillo, CA); Thr183Tyr185 of p42/p44 (ERK1/2) (Promega, Madison, WI); and pY31 paxillin (Invitrogen, Carlsbad, CA) visualized by enhanced chemiluminescence (ECL, Amersham, Arlington Heights, IL) and quantified by laser densitometry. Immunostained images (Nikon Microphot-FXA) were digitally captured with a Spot RT CCD (Diagnostic Instruments) [11].
Data Analysis
All values are mean ± SEM, with n = 4 or more. Data were analyzed using two-way ANOVA. Differences among means were considered significant at p<0.05. Data were analyzed using GraphPad statistical software.
Results and Discussion
FAK nuclear translocation
The subcellular localization in control NRVM of FAK seen with anti-FAK antibody had a punctuate pattern representing sites of focal adhesions but was entirely absent from the nuclei (Figure 1C). However, after 30 minutes of 10% static strain FAK was redistributed to spots within and around the nuclei (Figure 1D). Strained myofibrils were stained but not in control (Figure 1).
Nuclear-cytoplasmic shuttling may be a mechanism by which the rapid translocation of FAK from the cytoplasm to the nucleus and perinuclear region occurs. FAK and ERK1/2 phosphorylation initiate the Ras/MAPK pathway that dissociates a cytoplasmic complex enabling entry to the nucleus for hypertrophic gene expression [13-14]. Many other focal adhesion-associated proteins shuttle to the nucleus, including zyxin, paxillin and muscle LIM protein [14-15] where they regulate chromatin structure, transcription, mRNA processing and export. Interestingly, FAK accumulates in myocytes of failing hearts of spontaneously hypertensive rats [14].
Stretch-Induced FAK, ERK and paxillin activation in myocytes
The time course of FAK phosphorylation in response to 10% static stretch (Figure 2A) reached a peak of 47% at 5 min and remained elevated for 30 minutes (Figure 2B). The time course of ERK1/2 phosphorylation in response to 10% static stretch peaked at 1 min at 50% and decreased by 30 minutes (Figure 2C,D). A single 10% static strain resulted in a 40% increase in the level of pY31-paxillin, which peaked at 15 min and decreased by 30 min (p<0.05) (Figure 2E,F). These time courses are similar but the magnitude was lower than seen after brief endothelin and other stimulation [16]. Changes in FAK, ERK and paxillin protein expression levels were neither expected nor found since times are too brief for any significant increase in translation to occur.
Figure 2. Stretch-Induced FAK, ERK and paxillin.
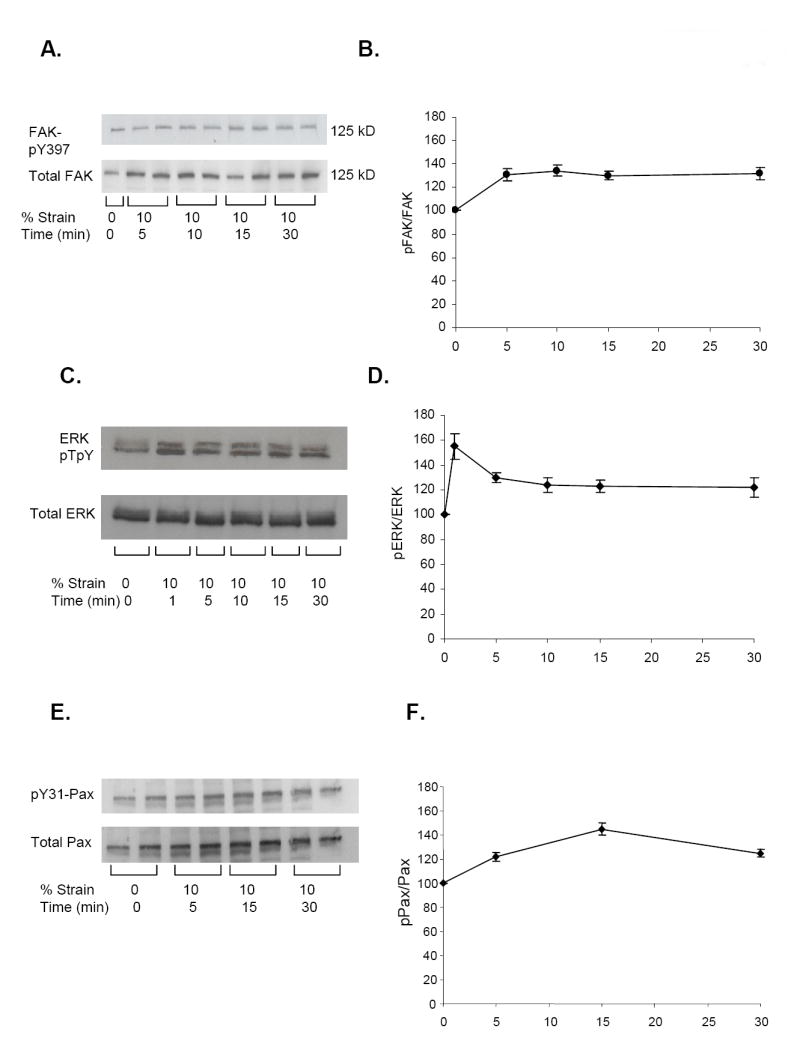
NRVM 10% static strain for 1-30 minutes. (A, C, E) Western blots: anti-FAKpY397, anti-ERKp42/44, anti-pY31; (B, C, E) Time courses normalized to controls: pFAK/FAK (n=4, *p<0.04); pERK1/2/ERK1/2 (n=4, *p<0.04); pPax/Pax (n=4, *p<0.05).
Chemical stimulation may activate different signaling pathways than a single mechanical strain. Vascular endothelial cells are continuously exposed to both mechanical and chemical stimuli. Mechanical (shear) and chemical (VEGF) stimuli diverge at the VEGF receptor 2 (Flk-1) and employ different components of the complex signaling network in regulating downstream molecules, such as ERK [17]. This may explain the difference in the peak activation levels of phosphorylation for FAK and ERK1/2 between mechanical or chemical stimuli.
Refractory period in FAK and ERK activation
Phosphorylation levels were assessed for different intervals between two 10%strains 5 or 15 mins apart (Figure 3A, B). There was no further response of Y397pFAK phosphorylation (p>0.07) for the second 5 min strain (Figure 3C) but the second 15 min strain was significantly increased by 30% (p<0.03), (Figure 3D). There was no further response of pERK1/2 phosphorylation (p>0.07) for the second 5 min strain but the second 15 min strain significantly increased the level of ERK1/2 phosphorylation by 50% (p<0.03), (Figure 3 EF).
Figure 3. Refractory period in FAK and ERK.
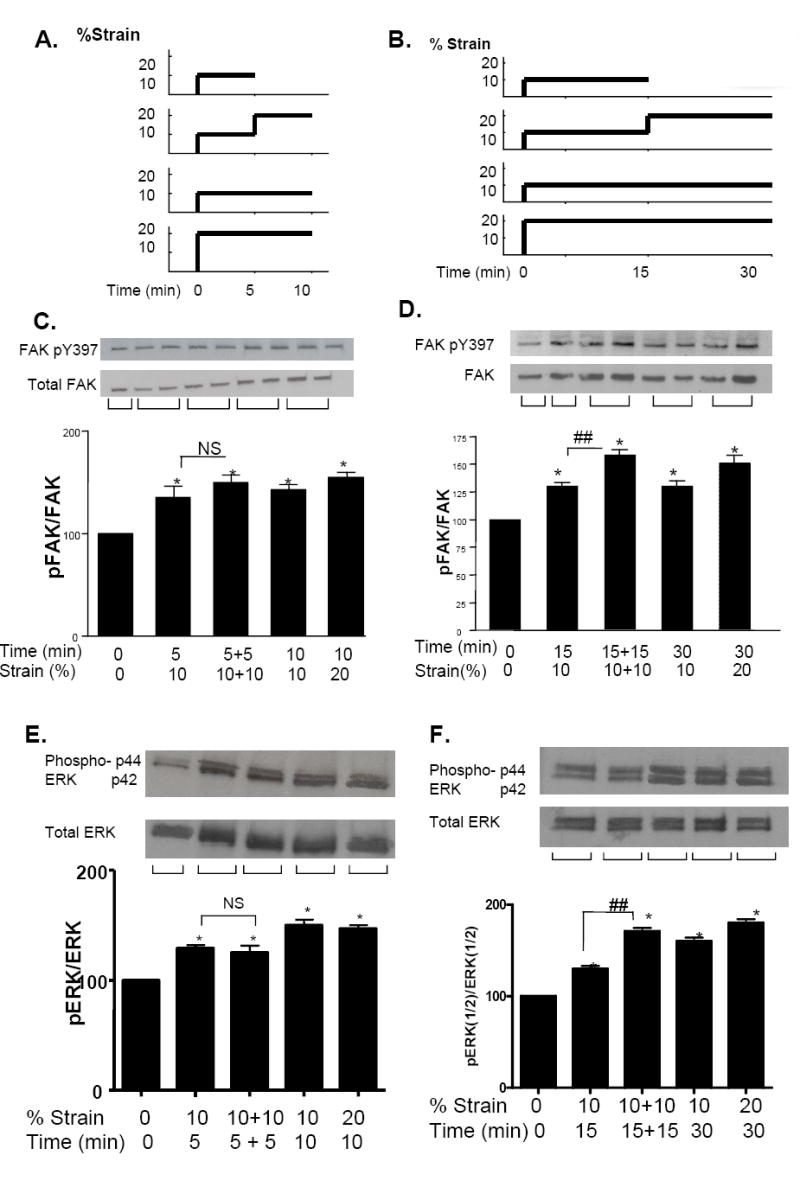
(A, B) Protocols for 10% strain delivery and maintenance. (C, D) Western blots and histograms: Y397pFAK/FAK (5 mins), (5+5 mins); (15+15 mins). (E, F) Western blots and histograms: ERK1/2 (5 mins); (5+5 mins); (15+15 mins). N=4, * p<0.05 normalized to control, ## p<0.05 between the first and second strain.
Refractory periods are common in physiology and describe the period of time during which an organ or cell is incapable of response, such as action potential initiation of nerves. Here, a second strain at the 5-minute interval yielded no further response although the 15-minute interval significantly increased phosphorylation levels for both FAK and ERK1/2. Thus, a second 10% strain yields 20% total strain that induces FAK and ERK higher phosphorylation (Fig 3), that is not realized when two steps are given separately at the 5-min interval of non-responsiveness but are detectable by 15-mins. This time scale fits with the time course of peak phosphorylation followed by the subsequent dephosphorylation of the different proteins (Figure 2B,D) [16]. Results suggest that mechanotransduction in cardiomyocytes has a refractory period of at least 5 minutes during which a new dynamic intervention might not be detected in a normal manner. Note that NRVM beat spontaneously throughout static stretch correlating to a sudden change of chamber volume in the heart that continues to beat as it adapts to a sudden extension of myocyte length.
Strain Rate Discrimination of frequency-dependent FAK and ERK1/2 Phosphorylation
Increased phosphorylation of FAK397 and ERK1/2pTEpY were found with increased rate of strain produced by higher cyclic frequency for biaxial perturbation of 10% strain for 20 minutes (Fig 4). Western blotting for FAK after strain rates of ~10% s-1, ~20% s-1, and ~40% s-1 induced 75%, 114%, and 125% of baseline, respectively, with statistical significance (p<0.05), (Figure 4A). The drop in phosphorylation at the lower frequency was surprising (Fig 4B, C). Similar strain rates correlated with a rapid increase in ERK1/2pTEpY of 87%, 290%, and 355%, respectively, significantly greater than baseline (p<0.05) (Fig 4D, E).
Figure 4. Frequency dependent phosphorylation.

(A) Cyclic biaxial strain rate for 20 min; slow 0.5Hz (~10% s-1), medium 1.0Hz (~20% s-1), and fast 2Hz (~ 40% s-1). (B, C) Western blot and histogram; FAK pY397 (10% s-1 vs. 40% s-1, ## p<0.05, n=7). (D,E) ERK pTEpY/ERK (n=4; *p<0.05).
ERK1/2 phosphorylation was detectable at 2min for 40% s-1 (not shown), as expected given response for static strain (Fig 2D). The rate of loading regulates the transmittance of force and overall activation of proteins relative to inherent relaxation constants of the cellular architecture. Strain rate sensitivity in a biaxial system may resolve from viscoelastic properties and relaxation time constants of the cellular membrane, focal adhesive complex, and the cytoskeleton [18]. These time constants could reflect growth of focal adhesive complexes that is unlikely to occur in minutes, so that signaling pathways are a more likely explanation.
The magnitude of the FAK phosphorylation here was two- and three-fold lower for static and cyclic strain, respectively, than other reports [19]. Removal of antibiotic cocktail from culture media for 48 hrs prior to strain increased levels of Y397pFAK to 124% ± 12% (n=4). This is not surprising since streptomycin and its analogs (gentamicin and netilmicin) are common aminoglycosidic antibiotics that have been reported to block L-type Ca2+ channels and stretch-activated channels in cardiac, skeletal, and vascular smooth muscle. In addition streptomycin can block [Ca2+]i transients and contraction in unstretched preparations [20]. Additionally, stretch may elicit entry from calcium channel populations in a rate dependent manner. The density of myocytes in culture also affects FAK expression and basal phosphorylation and the high density NRVM (2×103 cells /mm2) used here yields a lower concentration of FAK protein and a higher level of basal FAK phosphorylation.
Strain Vector Discrimination by FAK and ERK1/2 phosphorylation
NRVM align within grooved substrates as shown with FAKpY397 stain in culture (Figure 1B). NRVMs stretched 20 minutes with 10% transverse strain had 25% higher FAKpY397 phosphorylation to total FAK (p<0.05) than unstretched controls (Fig 5). However, FAK phosphorylation level for longitudinal strain resembled the unstretched rest group. NRVMs stretched at 10% strain had pTEpY to total ERK (Figure 5 D,E) of 360% for transverse strain and 290% for longitudinal strain, both significantly higher than the unstretched NRVM (p<0.05), though not differ from each other (transverse vs. longitudinal, p<0.10).
Figure 5. Direction dependent phosphorylation by Western blot and histogram.

(A) 10% cyclic strain transverse or longitudinal. (B, C) Y397pFAK/FAK (*p<0.05 vs control ## p<0.05 transverse vs. longitudinal, n=4). (D,E) ERKpTEpY/ERK: trend for transverse strain greater than longitudinal (p<0.10). Both greater than control (* p<0.05, n=4).
The anisotropic geometry of the cardiac myocyte may allow for distinct pathways of force recognition and transmittance. Mechanical forces induce protein conformation altering the dynamic state of focal adhesive proteins [21] perhaps contributing to vector dependent phosphorylation of FAK or a FAK activating protein. Separate directional pathways are implicated by static transverse and longitudinal loading to activate stress induced-MAP kinase. Potentially longitudinal perturbation activates a separate pathway that also runs into ERK activation as shown in similar studies with elongation of tissue sections from murine diaphragm [22]. Interestingly, microtubule density is implicated in direction and frequency dependent changes in viscosity over 1 to 10Hz perturbation of single adult cardiac myocytes [18]. Subsequent conformational changes may rely in part on the protein orientation; thus force vectors aligned with mobile protein components have a greater probability to modulate protein structural arrangement. NRVM used here are spontaneously beating, thereby adding a dynamic factor potentially complicating the experiments since cross-bridge formation affects longitudinal tensile stiffness [18]. Nonetheless, results detected an anisotropy even though the spontaneous beating was present for strains delivered along both vectors suggesting that the intrinsic beating does not override the externally applied stimuli.
Conclusion
Results show a refractory period of at least five minutes for FAK phosphorylation and more sensitivity in the transverse direction. Results for asymmetric and temporal detection add another dimension of complexity to whole heart mechanical studies in addition to the well known chemical and the simple mechanical perturbation studies. Thus, myocytes detect both temporal and anisotropic force changes that may be a critical for adaptation and maintenance of cardiac function when the mechanical demands on the heart are undergoing dynamic transformations due to disease.
Acknowledgments
We thank Tejal Desai, Allen Samarel and Samuel Boateng for advice and encouragement. The work was supported by NIH (HL 64956, HL 077995, HL62426) and American Heart Association Fellowship 558225.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holmes JW. Candidate mechanical stimuli for hypertrophy during volume overload. J Appl Physiol. 2004;97(4):1453–60. doi: 10.1152/japplphysiol.00834.2003. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzen-Schmidt I, Schmid-Schonbein GW, Giles WR, McCulloch AD, Chien S, Omens JH. Chronotropic response of cultured neonatal rat ventricular myocytes to short-term fluid shear. Cell Biochem Biophys. 2006;46(2):113–22. doi: 10.1385/CBB:46:2:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson DG, Majeski M, Borg TK, Terracio L. Regulation of cardiac myocytes protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res. 1999;85:59–69. doi: 10.1161/01.res.85.10.e59. [DOI] [PubMed] [Google Scholar]
- 4.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci. 2006;103(44):16095–100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopalan SM, Flaim C, Bhatia SN, Hoshijima M, Knoell R, Chien KR, Omens JH, McCulloch AD. Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol Bioeng. 2003;81(5):578–87. doi: 10.1002/bit.10506. [DOI] [PubMed] [Google Scholar]
- 6.Camelliti P, Gallagher JO, Kohl P, McCulloch AD. Mcropatterned cell cultures on elastic membrane as an in vitro model of myocardium. Nat Protoc. 2006;1(3):1379–91. doi: 10.1038/nprot.2006.203. [DOI] [PubMed] [Google Scholar]
- 7.Bullard TA, Hastings JL, Davis JM, Borg TK, Price RL. Altered PKC expression and phosphorylation in response to the nature, direction, and magnitude of mechanical stretch. Can J Physiol Pharmacol. 2007;85(2):243–50. doi: 10.1139/y07-023. [DOI] [PubMed] [Google Scholar]
- 8.Samarel AM. Costameres, focal adhesions, and cardiomyocytes mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;289:H2291–H2301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- 9.Mansour H, de Tombe PP, Samarel AM, Russell B. Restoration of resting sarcomere length after uniaxial static strain is regulated by PKCε and FAK. Circ Res. 2004;94:642–649. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- 10.Boateng SY, Hartman TJ, Aluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblasts proliferation in cardiac myocytes by surface microtopography. American Journal of Physiology – Cell Physiology. 2003;285:C171–182. doi: 10.1152/ajpcell.00013.2003. [DOI] [PubMed] [Google Scholar]
- 11.Motlagh D, Hartman TJ, Desai TA, Russell B. Microfabricated grooves recapitulate neonatal myocyte connexin43 and N-cadherin expression and localization. J Biomed Mater Res A. 2003;67(1):148–57. doi: 10.1002/jbm.a.10083. [DOI] [PubMed] [Google Scholar]
- 12.Sadoshima S, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–71. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 13.Wang JG, Miyazu M, Xiang P, Li SN, Sokabe M, Naruse K. Stretch-induced cell proliferation is mediated by FAK-MAPK pathway. Life Sci. 2005;76(24):2817–25. doi: 10.1016/j.lfs.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 14.Yi XP, Zhou J, Huber L, Qu J, Wang X, Gerdes AM, Li F. Nuclear compartmentalization of FAK and FRNK in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2006;290:H2509–H2515. doi: 10.1152/ajpheart.00659.2005. [DOI] [PubMed] [Google Scholar]
- 15.Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, de Tombe PP, Russell B. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am J Physiol Heart Circ Physiol. 2007;292:H259–69. doi: 10.1152/ajpheart.00766.2006. [DOI] [PubMed] [Google Scholar]
- 16.Heidkamp MC, Bayer AL, Scully BT, Eble DM, Samarel AM. Activation of focal adhesion kinase by protein kinase C in neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H1684–H1696. doi: 10.1152/ajpheart.00016.2003. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Chang J, Chen KD, Li S, Li JY, Wu C, Chien S. Selective adapter recruitment and differential signaling networks by VEGF vs. shear stress. Proc Natl Acad Sci U S A. 2007;104(21):8875–9. doi: 10.1073/pnas.0703088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura S, Nagai S, Katoh M, Yamashita H, Saeki Y, Okada J, Hisada T, Nagai R, Sugiura S. Microtubules modulate the stiffness of cardiomyocytes against shear stress. Circ Res. 2006;98(1):81–7. doi: 10.1161/01.RES.0000197785.51819.e8. [DOI] [PubMed] [Google Scholar]
- 19.Torsoni AS, Constancio SS, Nadruz W, Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res. 2003;93:140–147. doi: 10.1161/01.RES.0000081595.25297.1B. [DOI] [PubMed] [Google Scholar]
- 20.Belus A, White E. Streptomycin and intracellular calcium modulate the response of single guinea-pig ventricular myocytes to axial stretch. J Physiol. 2003;546(pt 2):501–9. doi: 10.1113/jphysiol.2002.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–88. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Chaudhry I, Reid MB, Boriek AM. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J Biol Chem. 2002;277(48):46493–503. doi: 10.1074/jbc.M203654200. [DOI] [PubMed] [Google Scholar]


