Abstract
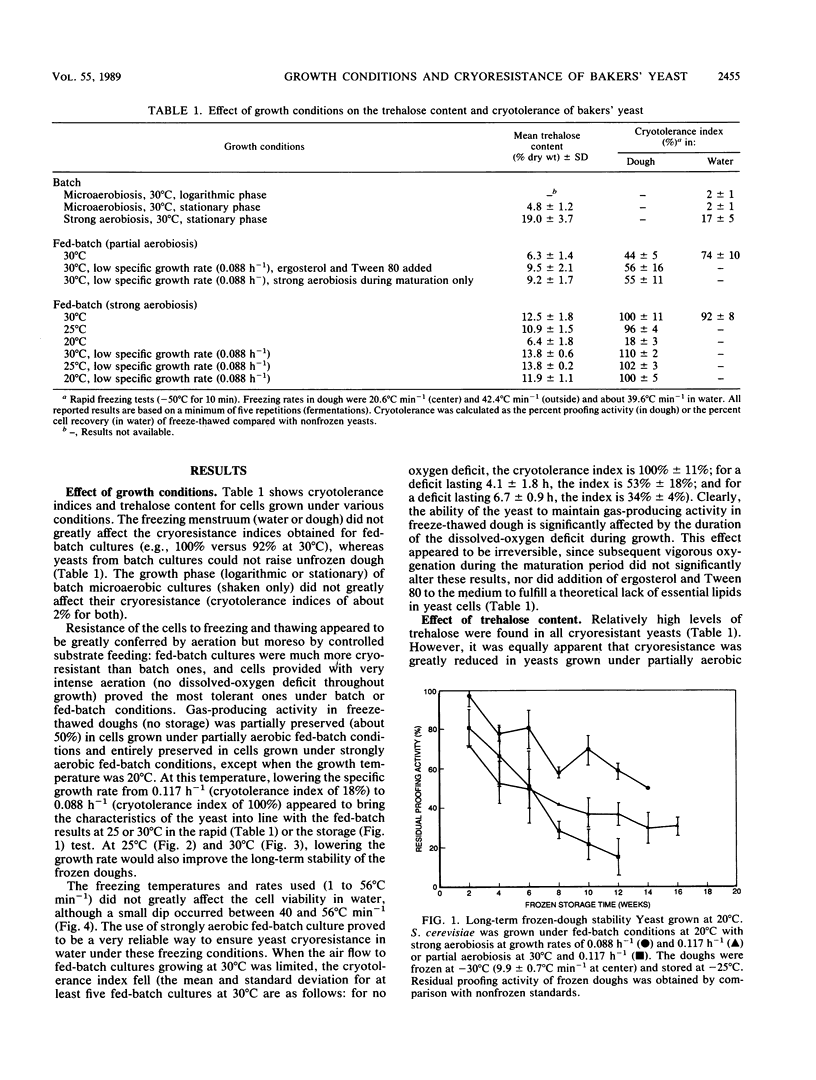
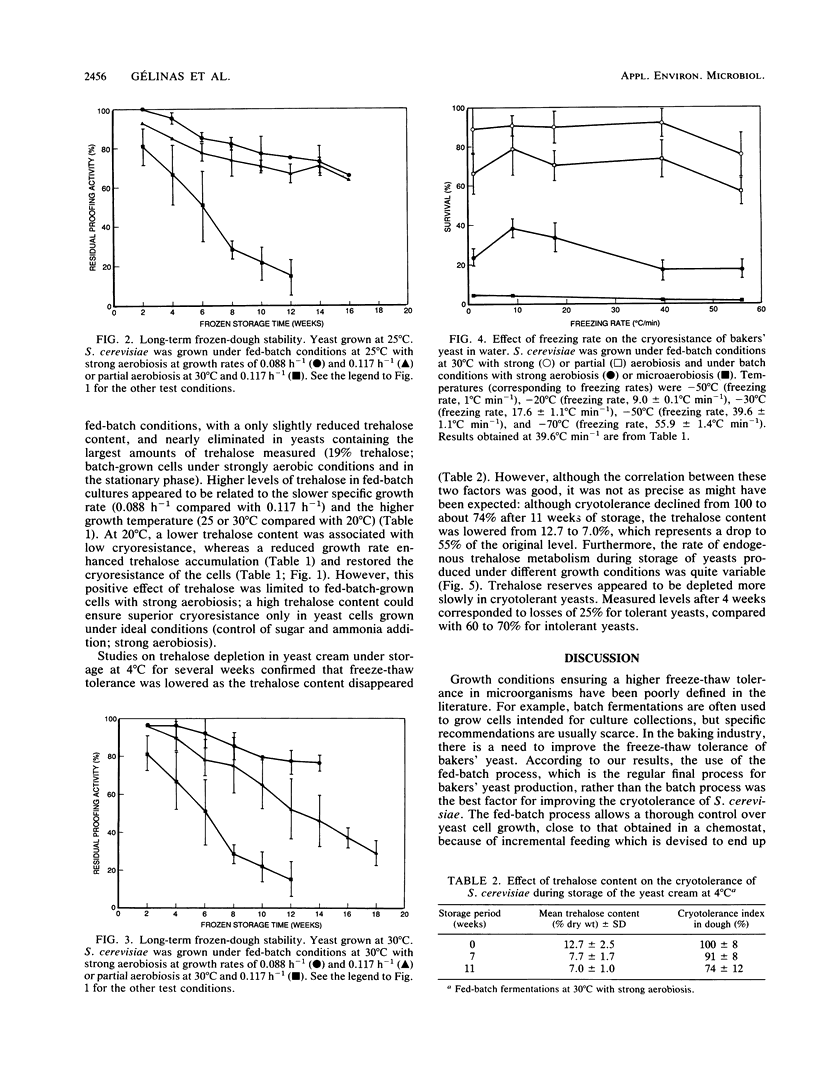
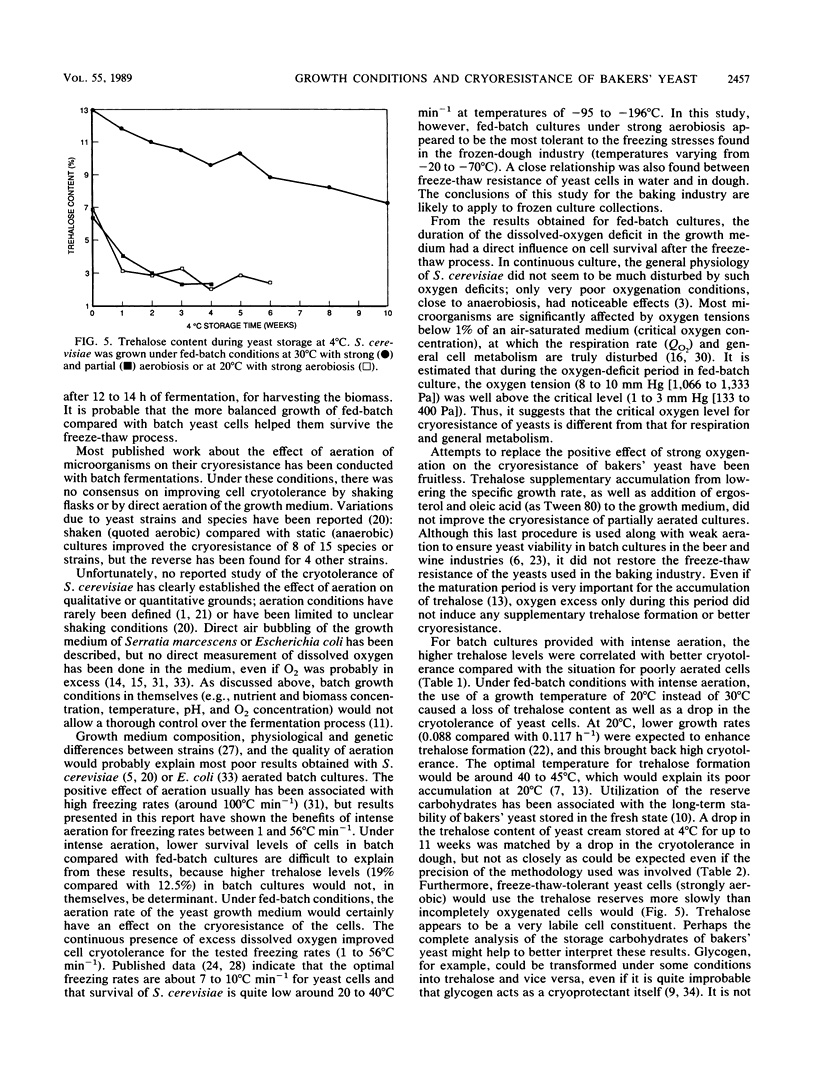
The cryotolerance in frozen doughs and in water suspensions of bakers' yeast (Saccharomyces cerevisiae) previously grown under various industrial conditions was evaluated on a laboratory scale. Fed-batch cultures were very superior to batch cultures, and strong aeration enhanced cryoresistance in both cases for freezing rates of 1 to 56°C min−1. Loss of cell viability in frozen dough or water was related to the duration of the dissolved-oxygen deficit during fed-batch growth. Strongly aerobic fed-batch cultures grown at a reduced average specific rate (μ = 0.088 h−1 compared with 0.117 h−1) also showed greater trehalose synthesis and improved frozen-dough stability. Insufficient aeration (dissolved-oxygen deficit) and lower growth temperature (20°C instead of 30°C) decreased both fed-batch-grown yeast cryoresistance and trehalose content. Although trehalose had a cryoprotective effect in S. cerevisiae, its effect was neutralized by even a momentary lack of excess dissolved oxygen in the fed-batch growth medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown C. M., Johnson B. Influence of oxygen tension on the physiology of Saccharomyces cerevisiae in continuous culture. Antonie Van Leeuwenhoek. 1971;37(4):477–487. doi: 10.1007/BF02218518. [DOI] [PubMed] [Google Scholar]
- Calcott P. H., MacLeod R. A. Survival of Escherichia coli from freeze-thaw damage: influence of nutritional status and growth rate. Can J Microbiol. 1974 May;20(5):683–689. doi: 10.1139/m74-104. [DOI] [PubMed] [Google Scholar]
- Casey G. P., Magnus C. A., Ingledew W. M. High-gravity brewing: effects of nutrition on yeast composition, fermentative ability, and alcohol production. Appl Environ Microbiol. 1984 Sep;48(3):639–646. doi: 10.1128/aem.48.3.639-646.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON A. P., Jr, CERRONI R. E. Fallacy of crushing death in frozen bacterial suspensions. Proc Soc Exp Biol Med. 1956 Apr;91(4):577–579. doi: 10.3181/00379727-91-22334. [DOI] [PubMed] [Google Scholar]
- HARRISON A. P., Jr Survival of bacteria upon repeated freezing and thawing. J Bacteriol. 1955 Dec;70(6):711–715. doi: 10.1128/jb.70.6.711-715.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F., Schellenberg M., Wiemken A. Localization of trehalase in vacuoles and of trehalose in the cytosol of yeast (Saccharomyces cerevisiae). Arch Microbiol. 1982 Jun;131(4):298–301. doi: 10.1007/BF00411175. [DOI] [PubMed] [Google Scholar]
- Kruuv J., Lepock J. R., Keith A. D. The effect of fluidity of membrane lipids on freeze-thaw survival of yeast. Cryobiology. 1978 Feb;15(1):73–79. doi: 10.1016/0011-2240(78)90009-3. [DOI] [PubMed] [Google Scholar]
- Küenzi M. T., Fiechter A. Regulation of carbohydrate composition of Saccharomyces cerevisiae under growth limitation. Arch Mikrobiol. 1972;84(3):254–265. doi: 10.1007/BF00425203. [DOI] [PubMed] [Google Scholar]
- Larue F., Lafon-Lafourcade S., Ribereau-Gayon P. Relationship between the sterol content of yeast cells and their fermentation activity in grape must. Appl Environ Microbiol. 1980 Apr;39(4):808–811. doi: 10.1128/aem.39.4.808-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P., Schmidt J. J. Interactions of cooling velocity, temperature, and warming velocity on the survival of frozen and thawed yeast. Cryobiology. 1968 Jul-Aug;5(1):1–17. doi: 10.1016/s0011-2240(68)80138-5. [DOI] [PubMed] [Google Scholar]
- McBride M. J., Ensign J. C. Effects of intracellular trehalose content on Streptomyces griseus spores. J Bacteriol. 1987 Nov;169(11):4995–5001. doi: 10.1128/jb.169.11.4995-5001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y., Uno K., Ohta S. Selection of yeasts for breadmaking by the frozen-dough method. Appl Environ Microbiol. 1986 Oct;52(4):941–943. doi: 10.1128/aem.52.4.941-943.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKER E. L., INGRAHAM J. L., SCHER S. FACTORS AFFECTING THE RATE OF KILLING OF ESCHERICHIA COLI BY REPEATED FREEZING AND THAWING. J Bacteriol. 1965 Mar;89:718–724. doi: 10.1128/jb.89.3.718-724.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redway K. F., Lapage S. P. Effect of carbohydrates and related compounds on the long-term preservation of freeze-dried bacteria. Cryobiology. 1974 Feb;11(1):73–79. doi: 10.1016/0011-2240(74)90040-6. [DOI] [PubMed] [Google Scholar]
- Rudolph A. S., Crowe J. H. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology. 1985 Aug;22(4):367–377. doi: 10.1016/0011-2240(85)90184-1. [DOI] [PubMed] [Google Scholar]
- Strauss G., Schurtenberger P., Hauser H. The interaction of saccharides with lipid bilayer vesicles: stabilization during freeze-thawing and freeze-drying. Biochim Biophys Acta. 1986 Jun 13;858(1):169–180. doi: 10.1016/0005-2736(86)90303-2. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. 7. Yeast carbohydrate fractions. Separation from nucleic acid, analysis, and behaviour during anaerobic fermentation. Biochem J. 1956 May;63(1):23–33. doi: 10.1042/bj0630023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womersley C., Uster P. S., Rudolph A. S., Crowe J. H. Inhibition of dehydration-induced fusion between liposomal membranes by carbohydrates as measured by fluorescence energy transfer. Cryobiology. 1986 Jun;23(3):245–255. doi: 10.1016/0011-2240(86)90050-7. [DOI] [PubMed] [Google Scholar]


