Abstract
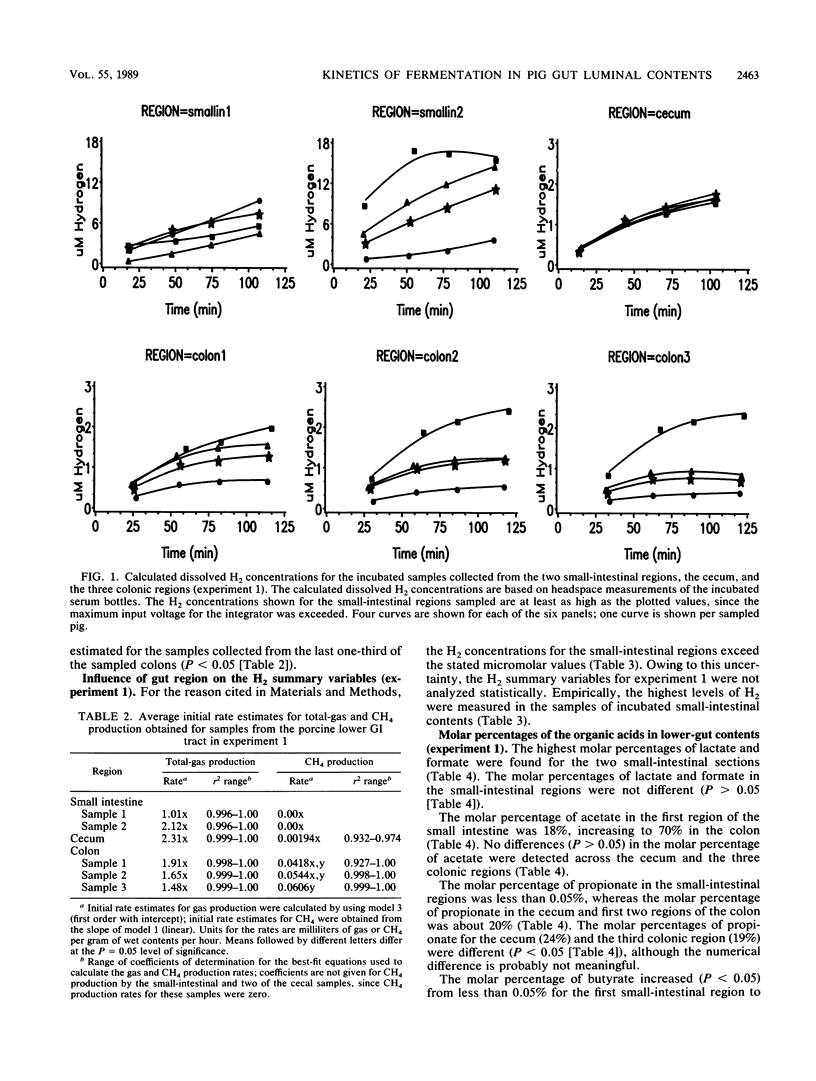
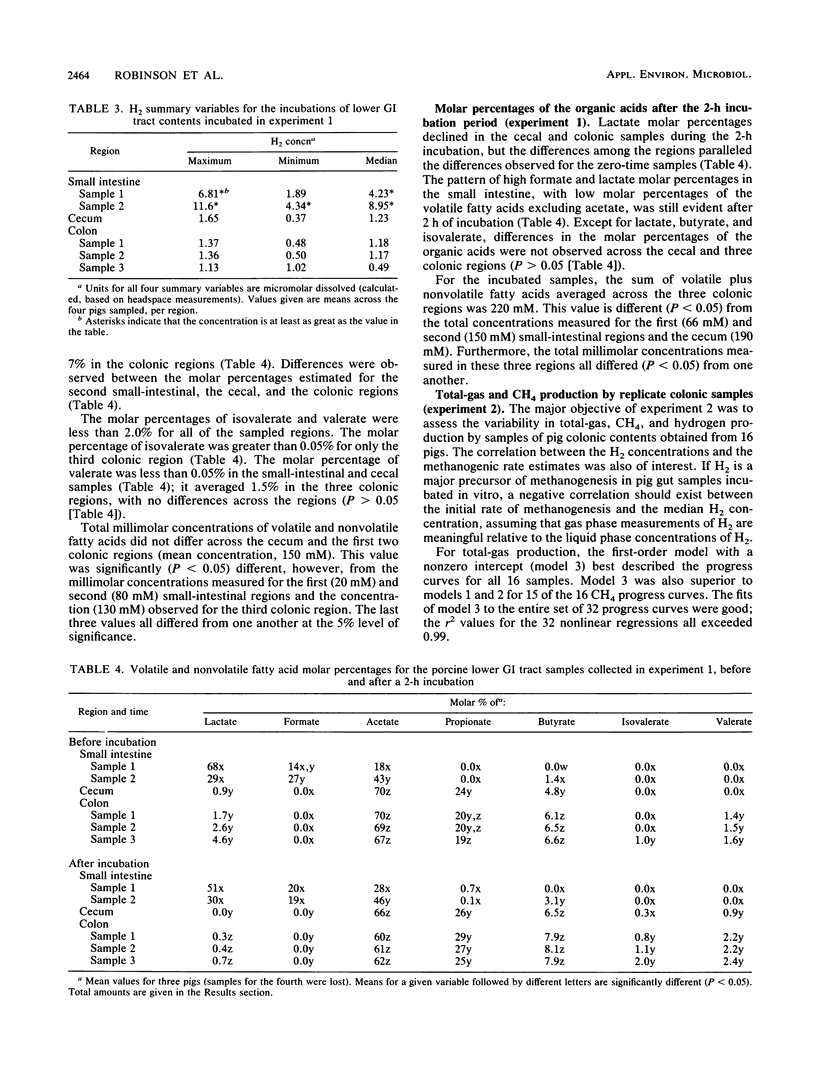
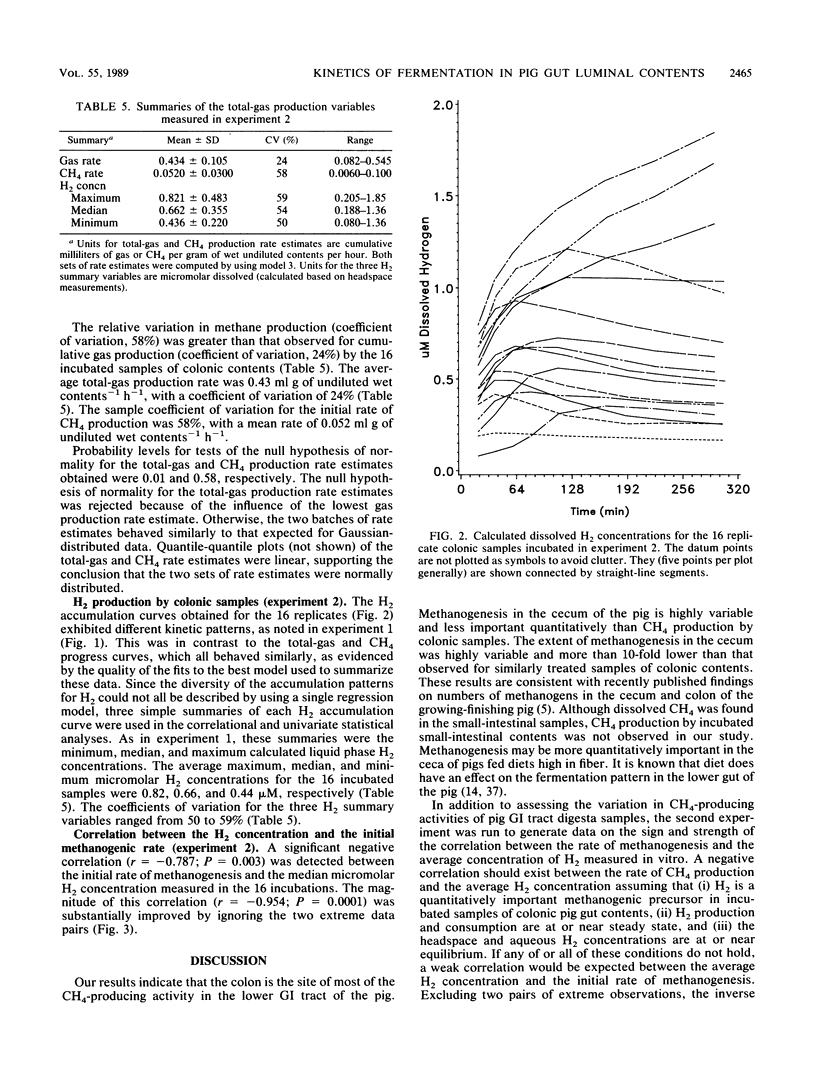
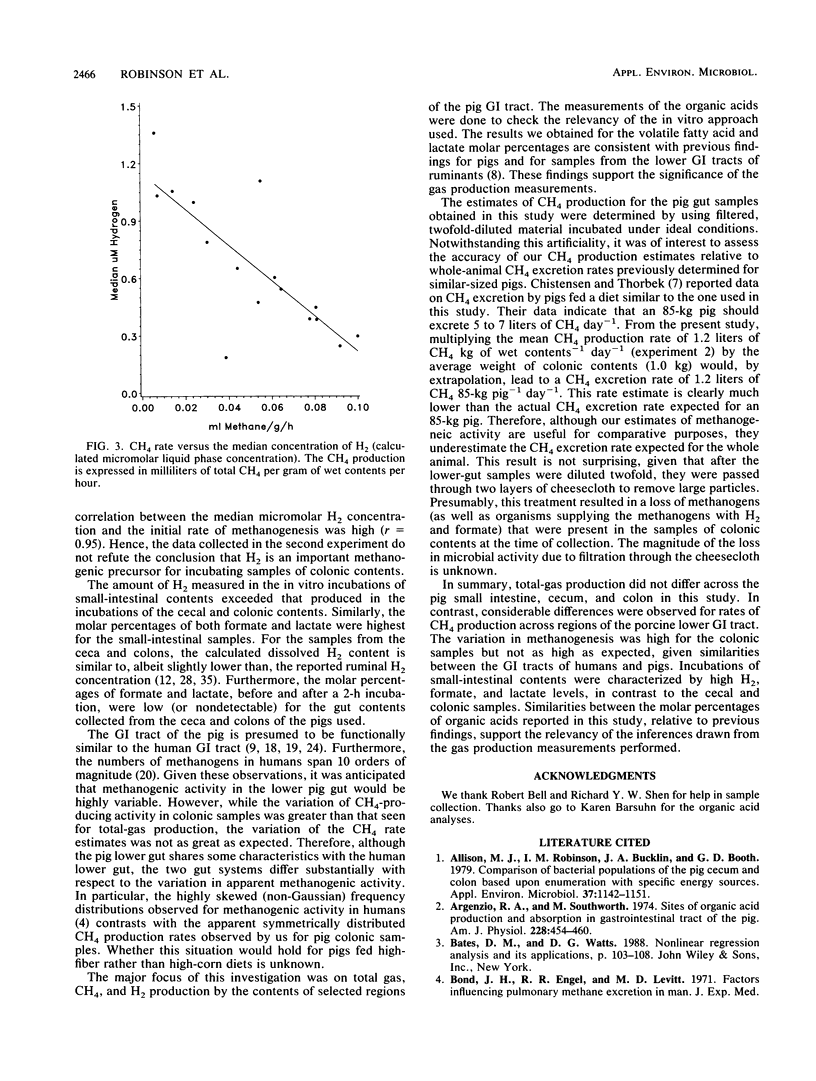
Two experiments were conducted to assess differences in fermentative activities of digesta obtained from various regions of the pig gastrointestinal tract. In experiment 1, the contents of small intestines, ceca, and colons of 110-kg pigs were collected, diluted twofold, and incubated for 2 h at 37 degrees C. In experiment 2, colonic samples from 16,100-kg pigs were similarly treated, except that the incubation period was 5 h. Total gas (gas pressure), CH4, H2, lactate, formate, acetate, propionate, butyrate, valerate, and isovalerate were measured in experiment 1. Only the gas variables were measured in experiment 2. Statistically significant differences (P greater than 0.05) were not observed among the gas production rate estimates across the small-intestinal, cecal, and colonic regions in experiment 1. Furthermore, all the small-intestinal samples and half the cecal samples assayed in experiment 1 were nonmethanogenic. The mean methanogenic and total-gas production rate estimates for the colonic samples in experiment 1 were 0.052 ml g of wet contents-1 h-1 and 1.7 ml of total gas g of wet contents-1 h-1, respectively. No differences in the methanogenic rate estimates were detected between the proximal, middle, and distal thirds of the pig colons (P greater than 0.05). The volatile fatty acid and lactate molar percentages measured in experiment 1 were consistent with previously published observations. Hydrogen accumulated to the greatest extent (7 microM on average) in the in vitro incubations of small-intestinal contents, whereas the H2 concentrations ranged from 0.5 to 1 microM for the incubated cecal and colonic samples in experiment 1.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J., Robinson I. M., Bucklin J. A., Booth G. D. Comparison of bacterial populations of the pig cecum and colon based upon enumeration with specific energy sources. Appl Environ Microbiol. 1979 Jun;37(6):1142–1151. doi: 10.1128/aem.37.6.1142-1151.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenzio R. A., Southworth M. Sites of organic acid production and absorption in gastrointestinal tract of the pig. Am J Physiol. 1975 Feb;228(2):454–460. doi: 10.1152/ajplegacy.1975.228.2.454. [DOI] [PubMed] [Google Scholar]
- Butine T. J., Leedle J. A. Enumeration of selected anaerobic bacterial groups in cecal and colonic contents of growing-finishing pigs. Appl Environ Microbiol. 1989 May;55(5):1112–1116. doi: 10.1128/aem.55.5.1112-1116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K., Thorbek G. Methane excretion in the growing pig. Br J Nutr. 1987 May;57(3):355–361. doi: 10.1079/bjn19870043. [DOI] [PubMed] [Google Scholar]
- Clemens E. T., Maloiy G. M., Sutton J. D. Molar proportions of volatile fatty acids in the gastrointestinal tract of East African wild ruminants. Comp Biochem Physiol A Comp Physiol. 1983;76(2):217–224. doi: 10.1016/0300-9629(83)90318-3. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Englyst H. N. Fermentation in the human large intestine and the available substrates. Am J Clin Nutr. 1987 May;45(5 Suppl):1243–1255. doi: 10.1093/ajcn/45.5.1243. [DOI] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Hungate R. E. Hydrogen as an intermediate in the rumen fermentation. Arch Mikrobiol. 1967;59(1):158–164. doi: 10.1007/BF00406327. [DOI] [PubMed] [Google Scholar]
- Imoto S., Namioka S. VFA production in the pig large intestine. J Anim Sci. 1978 Aug;47(2):467–478. doi: 10.2527/jas1978.472467x. [DOI] [PubMed] [Google Scholar]
- McNeil N. I. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984 Feb;39(2):338–342. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- Miller E. R., Ullrey D. E. The pig as a model for human nutrition. Annu Rev Nutr. 1987;7:361–382. doi: 10.1146/annurev.nu.07.070187.002045. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Moore L. V., Cato E. P., Wilkins T. D., Kornegay E. T. Effect of high-fiber and high-oil diets on the fecal flora of swine. Appl Environ Microbiol. 1987 Jul;53(7):1638–1644. doi: 10.1128/aem.53.7.1638-1644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J., Bucklin J. A. Characterization of the cecal bacteria of normal pigs. Appl Environ Microbiol. 1981 Apr;41(4):950–955. doi: 10.1128/aem.41.4.950-955.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. M., Whipp S. C., Bucklin J. A., Allison M. J. Characterization of predominant bacteria from the colons of normal and dysenteric pigs. Appl Environ Microbiol. 1984 Nov;48(5):964–969. doi: 10.1128/aem.48.5.964-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Strayer R. F., Tiedje J. M. Method for measuring dissolved hydrogen in anaerobic ecosystems: application to the rumen. Appl Environ Microbiol. 1981 Feb;41(2):545–548. doi: 10.1128/aem.41.2.545-548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. G. Types and distribution of anaerobic bacteria in the large intestine of pigs. Appl Environ Microbiol. 1979 Feb;37(2):187–193. doi: 10.1128/aem.37.2.187-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rérat A. Digestion and absorption of carbohydrates and nitrogenous matters in the hindgut of the omnivorous nonruminant animal. J Anim Sci. 1978 Jun;46(6):1808–1837. doi: 10.2527/jas1978.4661808x. [DOI] [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirhead P. A. Isolation and identification of fecal bacteria from adult swine. Appl Environ Microbiol. 1977 Jan;33(1):79–84. doi: 10.1128/aem.33.1.79-84.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanogias G., Pearce G. R. The digestion of fibre by pigs. 2. Volatile fatty acid concentrations in large intestine digesta. Br J Nutr. 1985 May;53(3):531–536. doi: 10.1079/bjn19850062. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Pond W. G., Pekas J. C., Yen J. T. Influence of high-fiber diet on bacterial populations in gastrointestinal tracts of obese- and lean-genotype pigs. Appl Environ Microbiol. 1982 Jul;44(1):107–112. doi: 10.1128/aem.44.1.107-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Robinson I. M., Jung H. J. Influence of dietary fiber on xylanolytic and cellulolytic bacteria of adult pigs. Appl Environ Microbiol. 1987 Jan;53(1):22–26. doi: 10.1128/aem.53.1.22-26.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


