Abstract
The activation of many tyrosine kinases leads to the phosphorylation and activation of phospholipase C-γ1 (PLC-γ1). To examine the biological function of this protein, homologous recombination has been used to selectively disrupt the Plcg1 gene in mice. Homozygous disruption of Plcg1 results in embryonic lethality at approximately embryonic day (E) 9.0. Histological analysis indicates that Plcg1 (−/−) embryos appear normal at E 8.5 but fail to continue normal development and growth beyond E 8.5–E9.0. These results clearly demonstrate that PLC-γ1 with, by inference, its capacity to mobilize second messenger molecules is an essential signal transducing molecule whose absence is not compensated by other signaling pathways or other genes encoding PLC isozymes.
Phosphoinositide-specific phospholipase C (PLC) activity catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate to the second messenger molecules inositol 1,4,5-trisphosphate and diacylglycerol. In mammalian cells, this hydrolytic activity is provoked by a substantial number of hormones and growth factors that regulate distinct PLC isozymes (1). To date, 10 PLC isozymes have been identified and classified into β, γ, and δ subfamilies based on overall sequence similarities and the conservation of sequence motifs X and Y that together form a catalytic domain. Hormones that signal through heterotrimeric G proteins modulate the activity of PLC-β isozymes. The PLC-γ isozymes uniquely contain two SH2 domains, which mediate association with receptor autophosphorylation sites, plus an SH3 domain of unclear function. Tyrosine kinase-dependent signaling pathways, such as growth factors and certain oncoproteins, phosphorylate and activate PLC-γ1, which is expressed ubiquitously. T and B cell receptors use soluble tyrosine kinases to activate PLC-γ1 as well as PLC-γ2, whose expression is most abundant in the lymphoid system. The mechanism by which PLC-δ isozymes are regulated is unclear.
The significance of PLC-γ1 as a signal transducing element in growth factor-initiated responses such as mitogenesis, chemotaxis, cell migration, and transformation in cell culture systems is ambiguous. For example, assays of growth factor-dependent mitogenesis have concluded that PLC-γ1 is either dispensable (2–4) or essential (5, 6). We chose to use the technology of targeted gene disruption to assess the biological function of PLC-γ1 within the context of the intact organism. Although disruption of genes encoding PLC isozymes related to the β and δ subtypes has been reported in lower organisms (7–10), there are no reports, as yet, of gene disruptions for PLC isozymes in mammals.
METHODS AND MATERIALS
PLC-γ1 Genomic DNA and Construction of Targeting Vectors.
A 129/SvJ mouse genomic DNA library (Stratagene) was screened using a l-kb mouse cDNA fragment that encodes both SH2 domains of PLC-γ1 (11) as a probe (a gift from B. Margolis, University of Michigan). Of ≈1 × 106 phage plaques screened, two overlapping clones encompassing 26.6 kb of Plcg1 genomic DNA were obtained. Sequencing of BamHI–BamHI subclones identified a 2.9-kb fragment containing exons corresponding to the X domain and both SH2 domains of rat PLC-γ1.
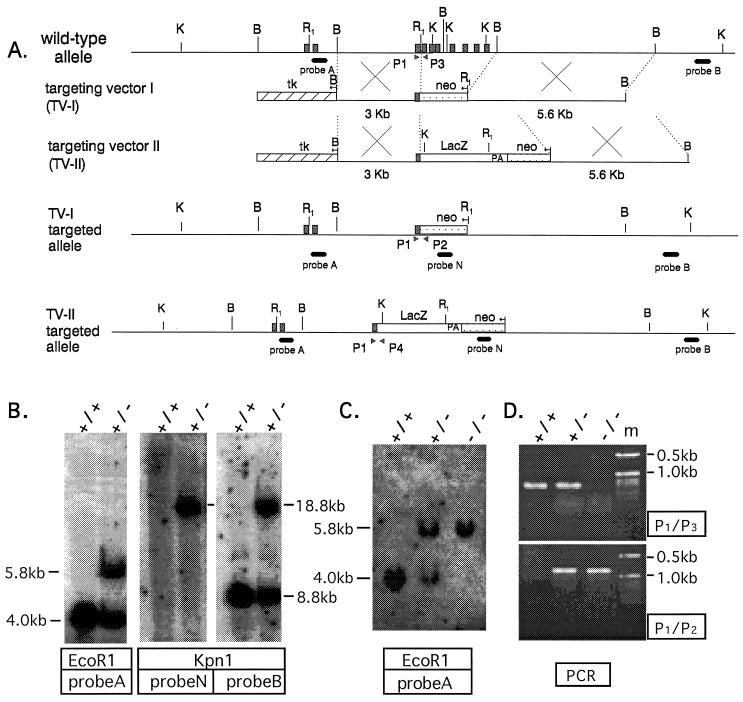
As depicted in Fig. 1A, targeting vectors (TV) I and II were constructed to contain 8.6 kb of 129/SvJ Plcg1 genomic DNA in the pPNT vector (12), which was modified by deletion of the EcoRI site. The 5′ homology region (3.0 kb) of the targeting vectors is derived from a BamHI–EcoRI Plcg1 genomic fragment that was subcloned into the BamHI–XbaI sites of the pPNT vector. The EcoRI site of this fragment was eliminated and replaced with an XbaI site before cloning into the pPNT vector. The 3′ homology region of the targeting vectors consisted of a 5.6-kb BamHI–BamHI fragment of Plcg1 genomic DNA that was first subcloned into pBlueScript II KS(−) (Stratagene). This fragment was then excised with XhoI–NotI and cloned into the XhoI–NotI sites of pPNT. Each targeting vector contained phosphoglycerate kinase neomycin resistance and phosphoglycerate kinase thymidine kinase polyadenylylate cassettes for positive and negative selection, respectively. The TV-II vector also contained an in-frame lacZ reporter sequence that, after recombination, encoded a β-galactosidase fusion protein with the amino terminus (residues 1–290) of PLC-γ1 and was expressed from the endogenous Plcg1 promoter.
Figure 1.
Gene targeting vectors, disruption strategy, and genotyping. (A) Genomic organization of Plcg1 with exons indicated by solid boxes. Also depicted are the targeting vectors TV-I and TV-II and the disrupted Plcg1 alleles for each targeting vector. B, BamHI; R1, EcoRI; K, KpnI. (B and C) Southern blots identifying wild-type and disrupted Plcg1 alleles after TV-I targeting of ES cells or embryos, respectively. (D) Representative PCR genotyping of Plcg1 alleles from embryos.
Transfection of Embryonic Stem (ES) Cells and Germ-Line Transmission.
TV-I or TV-II DNA was linearized with Not 1 and electroporated (3 μF and 800 mV) into R1 (13) or TL1 (14) ES cells, respectively. Approximately 106 cells were electroporated with 30 μg of DNA in a total volume of 800 μl. Transfected ES cells were selected in G418 (180 μg/ml) and gancyclovir (2 μM) for 7–10 days. To identify recombinant ES clones, Southern blotting with the 5′ external probe A (0.5 kb) after EcoRI digestion was used. Positive clones were verified by additional Southern blotting after KpnI digestion with external probe B (1.5 kb) and internal probe N (0.6 kb) derived from neomycin resistance. Transfection of R1 and TL1 ES cells with the TV-I and TV-II vectors, respectively, gave targeting frequencies of 3.6 and 11.9%, respectively. TV-I- and TV-II-derived heterozygous ES cells were microinjected into C57BL/6 blastocysts and implanted into pseudopregnant Institute for Cancer Research females. Resulting male chimeras were mated with female C57BL/6 mice. Germ-line transmission was identified in agouti offspring by Southern blotting for the disrupted Plcg1 allele, as described above.
Genotyping of Plcg1 Alleles.
Heterozygotes were interbred, and the offspring or embryos were analyzed for the wild-type and disrupted Plcg1 alleles by Southern blotting (as above) and/or PCR. PCR primers P1 (5′-GGCTGTCGACCGGCTCCAG-3′) and P3 (5′-ATCCAGCTGTGAGTTCCACAC-3′) were designed from Plcg1 exons 3 and 4 (Fig. 1A), respectively. Primer P2 (5′-CTATCAGGACATAGCGTTGGC-3′), representing the neomycin resistance cassette, and P4 (5′-ATTAAGTTGGGTAACGCCAGGG-3′), representing lacZ, also were used. Primer set P1/P3 detects the wild-type Plcg1 allele, and primer set P1/P2 or P1/P4 identifies Plcg1 alleles disrupted by TV-1 or TV-II, respectively.
Cell Culture and Immunoblot Analysis.
Mouse embryonic fibroblasts (MEF) were prepared according to standard methods (15) from day 9.5 embryos and were maintained in DMEM containing 10% fetal calf serum. To prepare extracts, cells were lysed in TGH buffer (1% Triton X-100/10% glycerol/50 mM Hepes, pH 7.2) containing 100 mM NaCl and protease and phosphatase inhibitors (10 μg/ml aprotinin/10 μg/ml leupeptin/0.1 mM phenylmethylsulfonyl fluoride/1 mM Na3VO4). After centrifugation at 4°C (16,000 × g, 10 min), soluble extracts were analyzed for PLC-γ1 by SDS/PAGE and Western blotting with or without prior immunoprecipitation. The antibody used for immunoprecipitation was specific for sequences (residues 1249–1262) in the carboxyl terminus of PLC-γ1 (16), and an antibody (Transduction Laboratories, Lexington, KY) specific for amino terminal sequences (residues 82–100) was used in Western blotting. Also, an antibody to β-galactosidase (Promega) was used to detect the TV-II fusion protein. Conditions for immunoprecipitation, SDS/PAGE, and Western blotting were as described (17). After Western blotting, bound antibodies were detected with anti-mouse IgG-conjugated horseradish peroxidase (Transduction Laboratories) and enhanced chemiluminescence (DuPont).
Embryo Morphology and PLC-γ1 Expression.
For histological analysis, embryos were fixed for 2 h with 4% paraformaldehyde in PBS at 4°C, dehydrated, and embedded in paraffin. The embryos were then sectioned (5–7 μm) and stained with hematoxylin and eosin. For β-galactosidase staining, embryos were fixed in 4% paraformaldehyde in PBS for 20 min and washed for 15 min with PBS containing 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40. Subsequently, the embryos were incubated at 37°C overnight with 2 mM MgCl2, 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, and 20 mM Tris (pH 7.4) with 1 mg/ml 5-bromo-4-chloro-3-indolyl β-d-galactoside (Sigma). Embryos were then washed in PBS and photographed or dehydrated in methanol.
RESULTS AND DISCUSSION
Gene Targeting.
Using a partial mouse PLC-γ1 cDNA (11) to screen a 129/SvJ mouse genomic library, two overlapping clones were isolated. Partial sequencing identified exons that were 95.3% and 49.6% identical at the protein level to corresponding rat sequences for PLC-γ1 and PLC-γ2, respectively. Rat cDNAs for PLC-γ1 and PLC-γ2 are 50.2% identical at the protein level (18). These results indicate that the isolated genomic clones represent Plcg1 and not Plcg2. In the mouse, these two genes are localized on different chromosomes (19, 23).
Targeted disruption of the Plcg1 gene was accomplished using replacement vectors TV-I or TV-II to delete genomic sequences encoding the X domain and both SH2 domains of PLC-γ1 (Fig. 1A). The X domain is essential for the catalytic activity of PLC-γ1 (1), and the SH2 domains are required for association of the enzyme with phosphotyrosine residues in activated tyrosine kinases as well as its subsequent tyrosine phosphorylation and activation. A representative Southern analysis of ES cells with wild-type and disrupted PLC-γ1 alleles is shown in Fig. 1B. Two independent ES cell clones derived with TV-I and one clone derived with TV-II then were used for blastocyst injections. Multiple chimeric animals were obtained for each blastocyst injection and produced germ-line transmission of the TV-I- and TV-II-disrupted Plcg1 alleles.
PLC-γ1 Null Animals.
The offspring of heterozygote crosses were examined for the appearance of the disrupted Plcg1 allele (Table 1). However, no Plcg1 (−/−) offspring were detected, and Plcg1 (+/+) and (+/−) offspring were obtained at the expected ratio (1:2) and were apparently normal. Embryos at various stages in development were then examined and genotyped by Southern blotting (Fig. 1C) or PCR (Fig. 1D). Midstage embryos [embryonic day (E) 11.5–13.5] yielded a small percentage of Plcg1 (−/−) embryos and a higher than expected proportion of empty decidua, suggestive of embryo resorption. In earlier (E 7.5–10.5) and preimplantation (E 3.0) embryos, the expected ratio of genotypes was recovered, with Plcg1 (−/−) embryos constituting 24% (Table 1). Hence, the disrupted Plcg1 allele is inherited in Mendelian fashion and Plcg1 (−/−) embryos die in midgestation.
Table 1.
Analysis of genotypes obtained from PLC-γ1 heterozygote crosses
| Stage | Total no.* | Genotype, %
|
Empty decidua, % | ||
|---|---|---|---|---|---|
| +/+ | +/− | −/− | |||
| Newborn offspring | 137 | 38 | 62 | 0 | — |
| Midstage embryos (E 11.5–13.5) | 53 | 23 | 51 | 9 | 17 |
| Early embryos (E 7.5–10.5) | 290 | 26 | 49 | 24 | 1 |
| Blastocysts (E 3.0) | 79 | 27 | 49 | 24 | — |
The totals represent both TV-I and TV-II disruptions of Plcg1 alleles and a compilation of the analyses of four mouse strains.
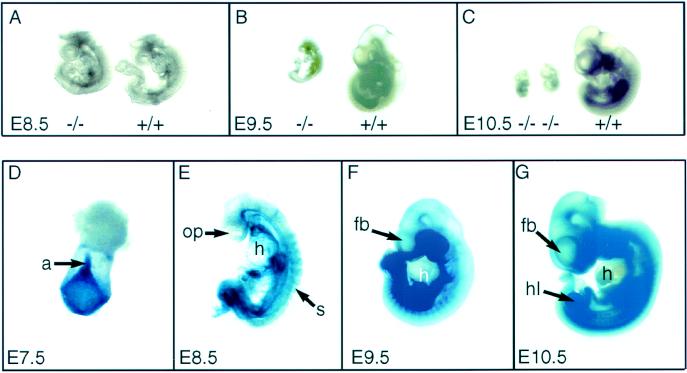
The gross morphology of Plcg1 (+/+) and (−/−) embryos at E 8.5–10.5 is shown in Fig. 2 (Upper). At E 8.5, most Plcg1 (−/−) embryos are of normal developmental appearance (Fig. 2A). However, a small fraction (≈20%) of null embryos did show evidence of retarded growth and development at this time (data not shown). By E 9.5, the growth and development of all Plcg1 (−/−) embryos were markedly retarded in size compared with wild-type embryos (Fig. 2B). By E 10.5, resorption of Plcg1 (−/−) embryos had begun (Fig. 2C), accounting for their low representation among midstage embryos at E 11.5–13.5 (Table 1). During development, Plcg1 (+/−) embryos were indistinguishable from wild-type embryos. We conclude that homozygous disruption of Plcg1 leads to embryonic lethality at approximately E 9.0–9.5. The lethal phenotype of plcg1 (−/−) embryos was consistent regardless of whether the Plcg1 gene was disrupted by TV-I or TV-II vectors in distinct ES cell lines. Also, the plcg1 (−/−) phenotype was not altered when the mutation was placed on different genetic backgrounds, namely inbred strains 129/SvJ or hybrid backgrounds 129/SvJ × C57BL/6, 129/SvJ × Black Swiss, and 129/SvJ × CD-1. Depicted Fig. 2 (Lower) are expression and localization of the Plcg1 gene product at E 7.5–10.5, as measured by β-galactosidase reporter staining in heterozygotes generated with the TV-II vector, which incorporates a lacZ reporter under regulation of the endogenous Plcg1 promoter. These data indicate that the Plcg1 gene is widely expressed in the embryo, with the highest levels in the first branchial arch, midline dorsal aorta, and limbs. No detectable β-galactosidase staining was observed in the forebrain, hindbrain, optic vesicle, and heart.
Figure 2.
Phenotype of Plcg1 (−/−) embryos and Plcg1 gene expression. (A–C) Morphologic appearance of Plcg1 (+/+) and (−/−) embryos at the indicated embryonic age. (D–G) Plcg1 gene expression, as judged by β-galactoside staining, of a Plcg1 (+/−) embryo derived by TV-II targeting at the indicated embryonic days. (D) Posterior view of the embryo. a, allantois; op, optic placode; s, somite; h, heart; fb, forebrain; hl, hindlimb.
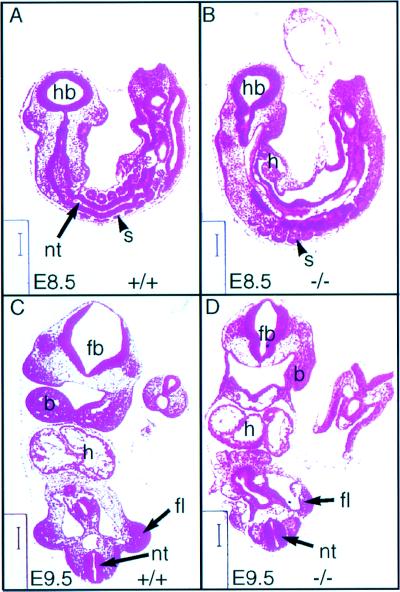
Histological sections of Plcg1 (+/+) and (−/−) embryos at E 8.5 and E 9.5 are shown in Fig. 3. These results show a comparable overall embryonic organization in both genotypes at both days. Although Plcg1 (−/−) embryos were considerably smaller at E 9.5, no specific abnormalities were detected at this level of examination. Neither placental growth nor maternal–fetal connections were markedly effected in Plcg1 (−/−) embryos (data not shown). Therefore, the cause of embryonic lethality due to homozygous Plcg1 disruption is not apparent.
Figure 3.
Histological analysis of Plcg1 wild-type and null embryos. Embryos at the indicated times were fixed, sectioned, and stained as described. hb, hindbrain; fb, forebrain; b, brachial arch; h, heart; nt, neural tube; s, somite. (Bar = 125 μm in A, 142 μm in B, 154 μm in C, and 111 μm in D.)
PLC-γ1 Null Fibroblasts.
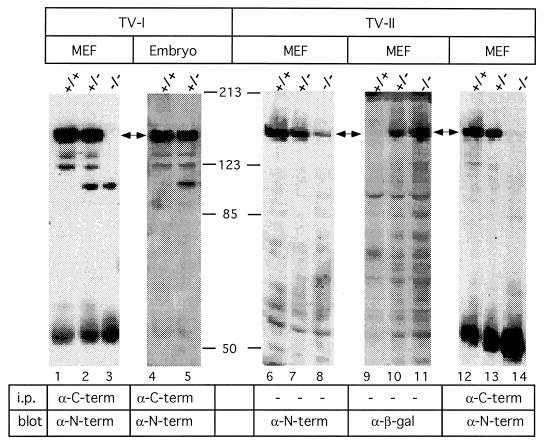
MEF were produced from Plcg1 (+/+) and (−/−) embryos and were assayed for the presence of PLC-γ1 protein. As shown in Fig. 4, PLC-γ1 (−/−) MEF from TV-I- (lane 3) or TV-II-targeted (lane 14) embryos do not contain the native (145-kDa) form of PLC-γ1, which reacts with antibodies to both amino and carboxyl termini (lanes 1, 2, 4, 5, 12, and 13). A small level of a 90-kDa form of PLC-γ1, however, was immunodetected in TV-I targeted (+/− and −/−) MEF and (+/−) embryos (lanes 2, 3, and 5). This protein represents ≈15% of the amount of native PLC-γ1 expressed in wild-type MEF. Given the size of this protein, its reactivity with antibodies to both the amino and carboxyl termini of PLC-γ1 and the gene targeting strategy used, this protein likely represents an aberrant form of PLC-γ1 produced by splicing, which encodes the amino terminus (≈290 residues before the X domain), SH3 domain, Y domain, and carboxyl terminus.
Figure 4.
Immunologic analysis of Plcg1 gene products. Cell lysates from MEF of the indicated Plcg1 genotypes were assayed with the indicated antibodies for the presence of reactive proteins as described. Each lane represents 300 μg of cell or embryo lysate. C-term, C terminus; N-term, N terminus; β-gal, β-galactosidase.
In MEF produced by TV-II targeting, a 145-kDa protein was detected by antibody to the PLC-γ1 amino terminus in all genotypes (lanes 6–8) and by antibody to β-galactosidase in Plcg1 (+/− and −/−) MEF (lanes 10–11) but not by antibody to the PLC-γ1 carboxyl terminus in Plcg1 (−/−) cells (lane 14). The 145-kDa protein detected in cells having a TV-II-disrupted allele had, based on the targeting strategy used, the expected size and immunoreactivity of the predicted fusion protein between the PLC-γ1 amino terminus and β-galactosidase.
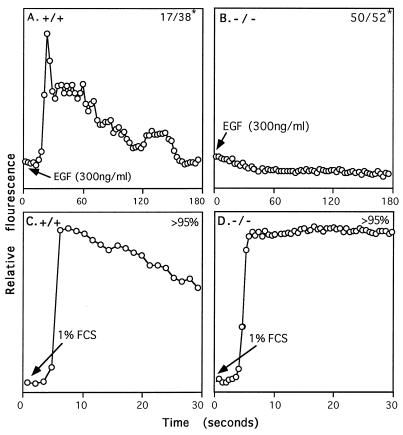
Although TV-I and TV-II targeting produced an identical phenotype in the animal, we tested the possibility that the 90-kDa form of PLC-γ1, detected in TV-I-targeted MEF and embryos, had functional activity for Ca2+ mobilization (Fig. 5). The results demonstrate that Plcg1 (−/−) MEF, produced by TV-I targeting, did not mobilize Ca2+ in response to epidermal growth factor. These null cells did mobilize Ca2+ in response to serum, which is accounted for by the activation of PLC-β isozymes in response to serum constituents, such as lysophosphatidic acid (20).
Figure 5.
Mobilization of intracellular Ca2+. Plcg1 wild-type and null MEF were seeded on coverslips and grown to near confluency before serum restriction (0.5% fetal calf serum) for 24 h. The coverslips were then washed with buffer (140 mM NaCl/5 mM KCl/1 mM MgCl2/10 mM Hepes, pH 7.4/0.55 M glucose), after which 1 μM fluo-3 AM (Molecular Probes) was added for 30 min at room temperature. The coverslips then were transferred to a Zeiss Axiovert 135 confocal microscope, and the fluorescence of individual cells was recorded at 488 nm after the addition of epidermal growth factor or fresh fetal calf serum. Numbers in the upper corner of each panel indicate responding cells per total number of cells examined. EGF, epidermal growth factor; FCS, fetal calf serum.
These results report, to our knowledge, the first functional analysis of a mammalian phosphoinositide-specific PLC in vivo and demonstrate the essential role of this signal-transducing molecule in the intact embryo. It seems likely that either the presence of PLC-γ1 is required for the proliferation of an essential embryonic cell type(s) that normally appears at approximately E 9.0 or that all cells fail to grow at a rate necessary for normal development of the embryo. The available data do not discern between these interpretations, which are being investigated further. Nevertheless, these results do demonstrate that the PLC-γ1 requirement in the embryo cannot be compensated for by other tyrosine kinase-initiated signaling pathways nor other PLC isozymes although available evidence indicates that the PLC-γ2 isozyme can respond to tyrosine kinase signals (21) and that, in some cells, PLC-β isoforms may be activated by tyrosine kinases (22).
Acknowledgments
We thank Drs. Brigid Hogan and David Piston for advice and assistance, Feng-lei Sun, Sandra Ermini, and Jill Lindner for technical assistance, and Sue Carpenter for manuscript preparation. This work was supported by National Institutes of Health Grants CA43720 (G.C.), CA64118 (R.W.), DK42502 (M.A.M.), and CA68485 (to the Vanderbilt Cancer Center). G.E.W. was supported by Brigid Hogan, an investigator of the Howard Hughes Medical Institute. K.D.N. is a Vanderbilt Medical Scientist Trainee (GM07347).
ABBREVIATIONS
- PLC
phospholipase C
- ES
embryonic stem
- TV
targeting vector
- MEF
mouse embryonic fibroblasts
- E
embryonic day
References
- 1.Noh D-Y, Shin S W, Rhee S G. Biochim Biophys Acta. 1995;1241:99–114. doi: 10.1016/0304-419x(95)00006-0. [DOI] [PubMed] [Google Scholar]
- 2.Peters K G, Marie J, Wilson E, Ives H E, Escobedo J, Del Rosario M, Mirda D, Williams L T. Nature (London) 1992;358:678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadi M, Dionne C A, Li W, Li N, Spivak T, Honegger A M, Jaye M, Schlessinger J. Nature (London) 1992;358:681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- 4.Rönnstrand L, Mori S, Arridsson A-K, Eriksson A, Wernstedt C, Hellman U, Claesson-Welsh L, Heldin C-H. EMBO J. 1992;11:3911–3919. doi: 10.1002/j.1460-2075.1992.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valius M, Bazenet C, Kazlauskas A. Mol Cell Biol. 1993;13:133–143. doi: 10.1128/mcb.13.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roche S, McGlade J, Jones M, Gish G D, Pawson T, Courtneidge S A. EMBO J. 1996;15:4940–4948. [PMC free article] [PubMed] [Google Scholar]
- 7.Bloomquist B T, Shortridge R D, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak W L. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 8.Yoko-o T, Matsui Y, Yagisawa H, Nojima H, Uno I, Toh-e A. Proc Natl Acad Sci USA. 1993;90:1804–1808. doi: 10.1073/pnas.90.5.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flick J S, Thorner J. Mol Cell Biol. 1993;13:5861–5876. doi: 10.1128/mcb.13.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drayer A L, Van der Kaay J, Mayr G W, Van Haastert P J M. EMBO J. 1994;13:1601–1609. doi: 10.1002/j.1460-2075.1994.tb06423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolis B, Silvennoinen O, Comoglio F, Roonprapunt C, Skolnik E, Ullrich A, Schlessinger J. Proc Natl Acad Sci USA. 1992;89:8894–8898. doi: 10.1073/pnas.89.19.8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tybulewicz V L J, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 13.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao G-Q, Deng K, Labosky P A, Liaw L, Hogan B L M. Genes Dev. 1996;10:1657–1669. doi: 10.1101/gad.10.13.1657. [DOI] [PubMed] [Google Scholar]
- 15.Todaro G J, Green H. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arteaga C L, Johnson M D, Todderud G, Coffey R J, Carpenter G, Page D L. Proc Natl Acad Sci USA. 1991;88:10435–10439. doi: 10.1073/pnas.88.23.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baulida J, Kraus M H, Alimandi M, Di Fiore P P, Carpenter G. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 18.Emori Y, Homma Y, Sorimachi H, Kawasaki H, Nakanishi O, Suzuki K, Takenawa T. J Biol Chem. 1989;264:21885–21890. [PubMed] [Google Scholar]
- 19.Nelson K K, Knopf J L, Siracusa L D. Mamm Genome. 1992;3:597–600. doi: 10.1007/BF00350627. [DOI] [PubMed] [Google Scholar]
- 20.Moolenaar W H. J Biol Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 21.Sultzman L, Ellis C, Lin L-L, Pawson T, Knopf J. Mol Cell Biol. 1991;11:2018–2025. doi: 10.1128/mcb.11.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W W, Mattingly R R, Garrison J C. Proc Natl Acad Sci USA. 1996;93:8258–8263. doi: 10.1073/pnas.93.16.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argeson A C, Druck T, Veronese M L, Knopf J L, Buchberg A M, Huebner K, Siracusa L D. Genomics. 1995;25:29–35. doi: 10.1016/0888-7543(95)80106-v. [DOI] [PubMed] [Google Scholar]