Abstract
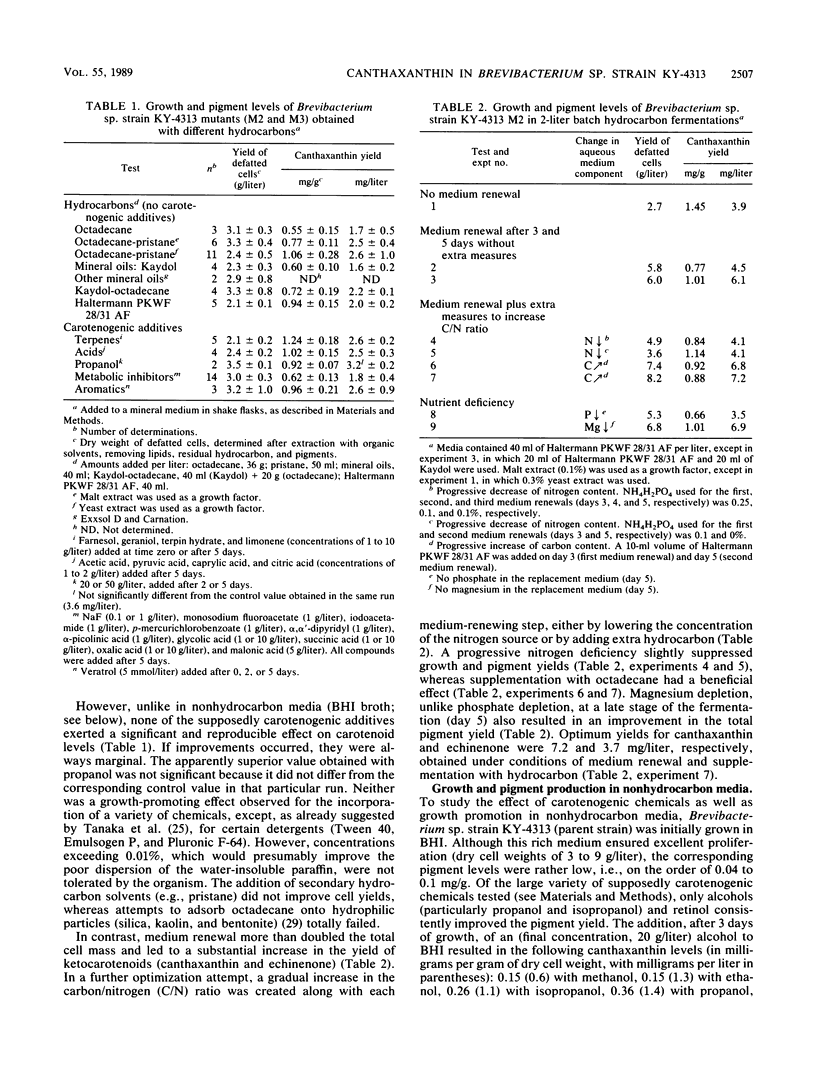
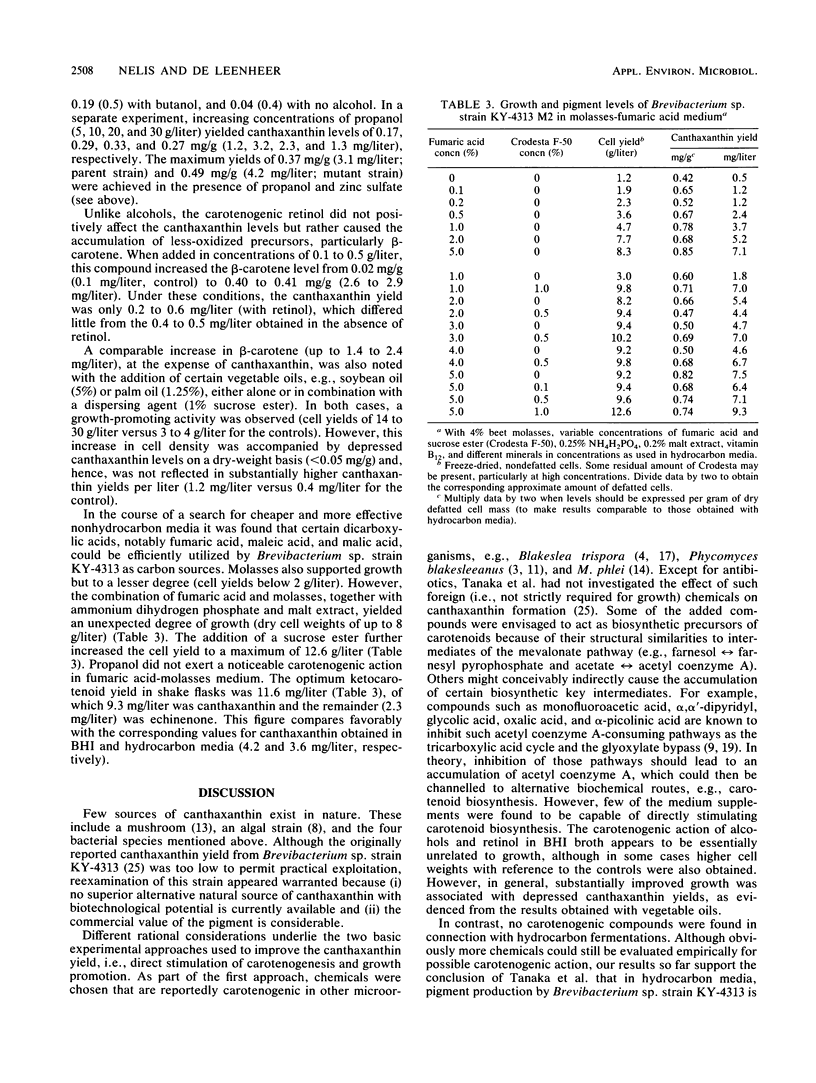
The hydrocarbon-utilizing Brevibacterium sp. strain KY-4313 was reevaluated for its potential to produce canthaxanthin, a carotenoid pigment of strong commercial interest. Three approaches were used to optimize the canthaxanthin yield from this organism, i.e., the preparation of mutants, the addition of supposedly carotenogenic chemicals to the growth medium, and growth promotion. Following treatment of the parent strain with N-nitrosomethylurea, a presumed mutant was isolated which showed a 32% increase in cellular canthaxanthin content. No effective carotenogenic chemicals were found in connection with hydrocarbon fermentations, in which mainly growth promotion through periodic medium renewal proved conducive to enhanced pigment production. Carotenogenesis could be stimulated in brain heart infusion broth by adding alcohols or retinol. Improved growth in this medium was generally not associated with higher canthaxanthin yields. Both superior growth and pigment levels were obtained in a newly designed medium based on fumaric acid-molasses. The maximum yields of canthaxanthin in shake flasks were (in milligrams per liter) 4.2 (brain heart infusion broth plus propanol-zinc sulfate), 3.6 (hydrocarbon medium), and 9.3 (fumaric acid-molasses), which represent a significant improvement over the originally reported optimal result (1 mg/liter). The corresponding yields of echinenone, the direct precursor of canthaxanthin, were 1.2, 1.6, and 2.3 mg/liter, respectively. Two-liter hydrocarbon batch fermentations involving medium renewal maximally produced 7.2 mg of canthaxanthin and 3.7 mg of echinenone per liter.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. H., Schuman D. B., Johnson E. A. Isolation of Phaffia rhodozyma Mutants with Increased Astaxanthin Content. Appl Environ Microbiol. 1989 Jan;55(1):116–124. doi: 10.1128/aem.55.1.116-124.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciegler A. Microbial carotenogenesis. Adv Appl Microbiol. 1965;7:1–34. doi: 10.1016/s0065-2164(08)70382-4. [DOI] [PubMed] [Google Scholar]
- Cooney J. J., Marks H. W., Smith A. M. Isolation and Identification of Canthaxanthin from Micrococcus roseus. J Bacteriol. 1966 Aug;92(2):342–345. doi: 10.1128/jb.92.2.342-345.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraseliya GYa Variability of Mycobacterium phlei in pigment production under the action of different mutagens. Sov Genet. 1973 Dec 1;7(8):1060–1063. [PubMed] [Google Scholar]
- Di Accadia F. D., Gribanovski-Sassu O., Romagnoli A., Tuttobello L. Isolation and identification of carotenoids produced by a green alga (Dictyococcus cinnabarinus) in submerged culture. Biochem J. 1966 Dec;101(3):735–740. doi: 10.1042/bj1010735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham M. A., Steenbock H. The relation of micro-organisms to carotenoids and vitamin A: The production of carotenoids by Mycobacterium phlei. Biochem J. 1935 Nov;29(11):2553–2562. doi: 10.1042/bj0292553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampila L. E., Wallen S. E., Bullerman L. B. A review of factors affecting biosynthesis of carotenoids by the order Mucorales. Mycopathologia. 1985 May;90(2):65–80. doi: 10.1007/BF00436853. [DOI] [PubMed] [Google Scholar]
- Ratledge C. Microbial conversions of n-alkanes to fatty acids: A new attempt to obtain economical microbial fats and fatty acids. Chem Ind. 1970 Jun 27;26:843–854. [PubMed] [Google Scholar]
- SAPERSTEIN S., STARR M. P. The ketonic carotenoid canthaxanthin isolated from a colour mutant of Corynebacterium michiganense. Biochem J. 1954 Jun;57(2):273–275. doi: 10.1042/bj0570273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungers G. E., Cooney J. J. Isolation and characterization of carotenoid pigments of Micrococcus roseus. J Bacteriol. 1968 Jul;96(1):234–241. doi: 10.1128/jb.96.1.234-241.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. ACTION OF MICROORGANISMS ON HYDROCARBONS. Bacteriol Rev. 1946 Mar;10(1-2):1–49. [PMC free article] [PubMed] [Google Scholar]


