Abstract
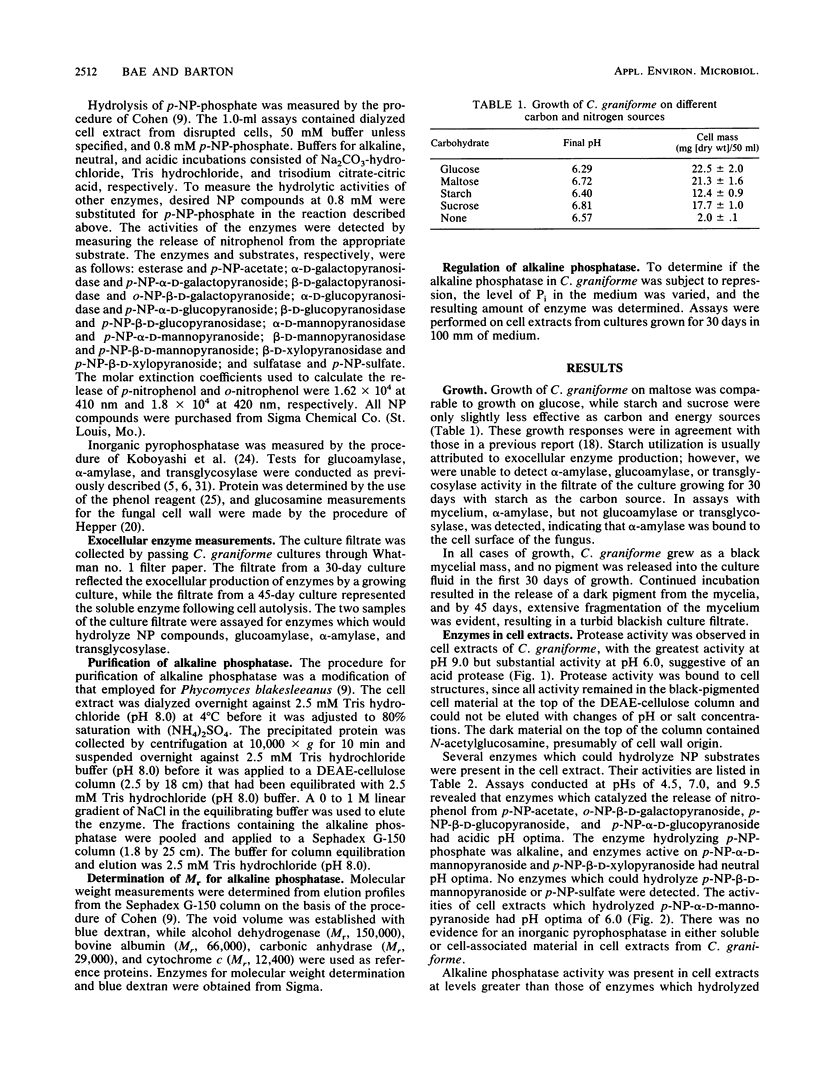
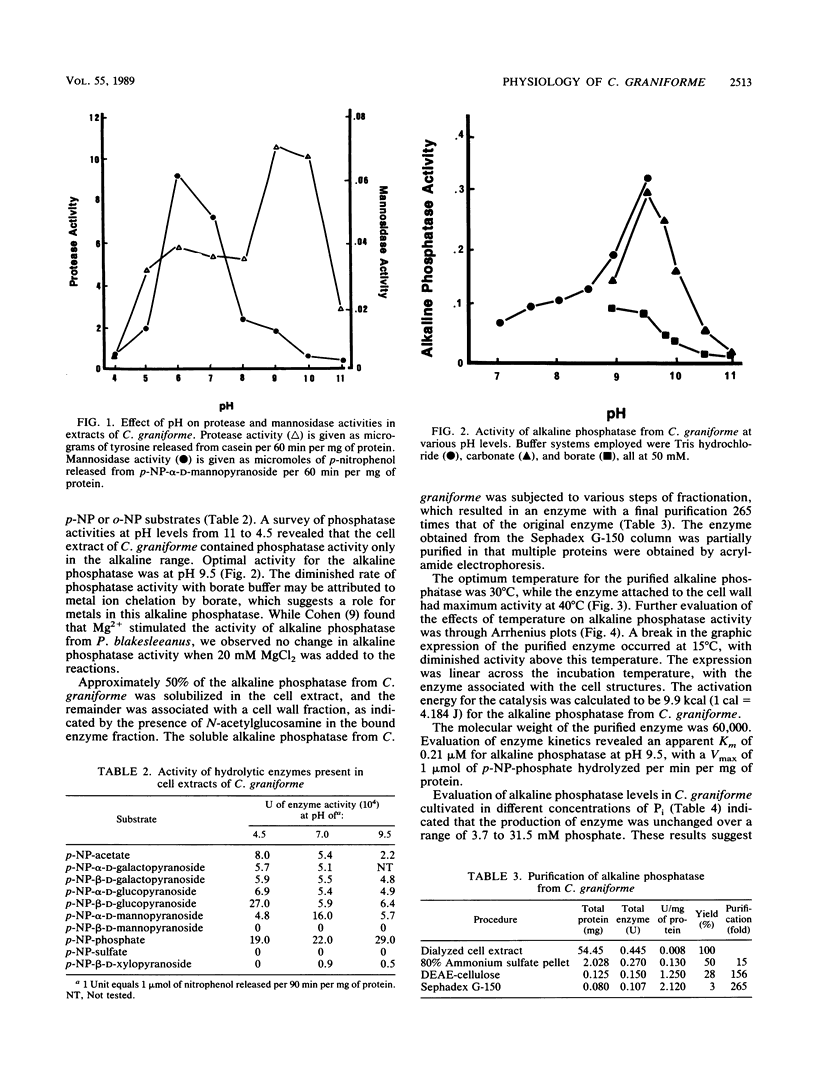
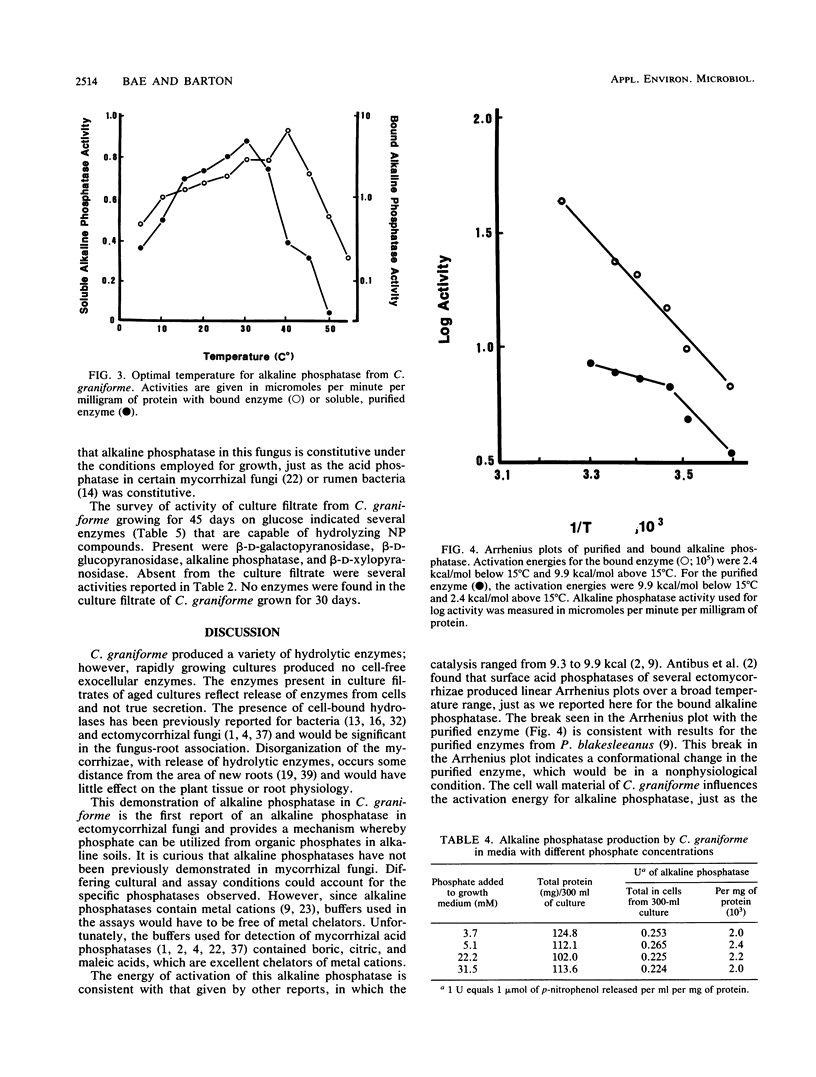
Cell extracts of Cenococcum graniforme have been found to contain the following hydrolytic enzymes: protease, esterase, α-d-galactopyranosidase, β-d-galactopyranosidase, α-d-mannopyranosidase, β-d-xylopyranosidase, α-d-glucopyranosidase, β-d-glucopyranosidase, and alkaline phosphatase. Sulfatase, inorganic pyrophosphatase, and β-d-mannopyranosidase were not detected in the extracts. β-d-Xylopyranosidase and α-d-mannopyranosidase were most active in the neutral pH range, protease and phosphatase were most active in the alkaline pH range, and other enzymes were most active in the acidic pH range. These enzymes showed a high association with cell wall material, and the release of enzymes from the cells into the culture fluid appeared to occur only when the cells were undergoing autolysis. Alkaline phosphatase in C. graniforme is a constitutive enzyme, and examination of the alkaline phosphatase following a purification of 265-fold produced the following characteristics: pH optimum of 9.5, Mr of 60,000, Km of 2.1 × 10-4 M for p-nitrophenylphosphate, and activation energy for hydrolysis of the substrate at 9.9 kcal (1 cal = 4.184 J)/mol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton L. L., Georgi C. E., Lineback D. R. Effect of maltose on glucoamylase formation by Aspergillus niger. J Bacteriol. 1972 Sep;111(3):771–777. doi: 10.1128/jb.111.3.771-777.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. W., Lees H. Alkaline phosphatases of Neurospora crassa. 3. Effects of pH and mechanism of action. Can J Microbiol. 1973 Jan;19(1):135–146. doi: 10.1139/m73-020. [DOI] [PubMed] [Google Scholar]
- Davis F. W., Lees H. Alkaline phosphatases of Neurospora crassa. II. Product inhibition studies. Can J Microbiol. 1972 Apr;18(4):407–421. doi: 10.1139/m72-065. [DOI] [PubMed] [Google Scholar]
- Day D. F., Ingram J. M. In vitro studies of an alkaline phosphatase-cell wall complex from Pseudomonas aeruginosa. Can J Microbiol. 1975 Jan;21(1):9–16. doi: 10.1139/m75-002. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Cheng K. J. The constitutive nature of alkaline phosphatase in rumen bacteria. Can J Microbiol. 1980 Feb;26(2):268–272. doi: 10.1139/m80-043. [DOI] [PubMed] [Google Scholar]
- Gadgil R. L., Gadgil P. D. Mycorrhiza and litter decomposition. Nature. 1971 Sep 10;233(5315):133–133. doi: 10.1038/233133a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Vallespir S., Ghosh B. K. Specificity of subcellular distribution of alkaline phosphatase in Bacillus licheniformis 749/C. Can J Microbiol. 1984 Jan;30(1):113–125. doi: 10.1139/m84-019. [DOI] [PubMed] [Google Scholar]
- Hochberg M. L., Sargent M. L. Regulation of repressible alkaline phosphatase by organic acids and metal ions in Neurospora crassa. Can J Microbiol. 1973 Dec;19(12):1487–1492. doi: 10.1139/m73-242. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Morisawa Y., Ishituka T., Ishimoto M. Biochemical studies on sulfate-reducing bacteria. XIV. Enzyme levels of adenylylsulfate reductase, inorganic pyrophosphatase, sulfite reductase, hydrogenase, and adenosine triphosphatase in cells grown on sulfate, sulfite, and thiosulfate. J Biochem. 1975 Nov;78(5):1079–1085. doi: 10.1093/oxfordjournals.jbchem.a130985. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCCONN J. D., TSURU D., YASUNOBU K. T. BACILLUS SUBTILIS NEUTRAL PROTEINASE. I. A ZINC ENZYME OF HIGH SPECIFIC ACTIVITY. J Biol Chem. 1964 Nov;239:3706–3715. [PubMed] [Google Scholar]
- PAZUR J. H., ANDO T. The isolation and the mode of action of a fungal transglycosylase. Arch Biochem Biophys. 1961 Apr;93:43–49. doi: 10.1016/0003-9861(61)90313-7. [DOI] [PubMed] [Google Scholar]
- Poirier T. P., Holt S. C. Acid and alkaline phosphatases of Capnocytophaga species. I. Production and cytological localization of the enzymes. Can J Microbiol. 1983 Oct;29(10):1350–1360. doi: 10.1139/m83-210. [DOI] [PubMed] [Google Scholar]
- Wilson R. W. Acid and alkaline phosphatases in Schizophyllum commune. Can J Microbiol. 1972 May;18(5):694–695. doi: 10.1139/m72-109. [DOI] [PubMed] [Google Scholar]



