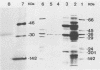
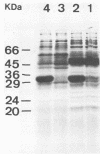
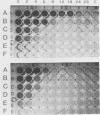
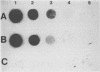
Abstract
As a system for studying the fate of genetically engineered microorganisms in the environment, we have previously constructed recombinant plasmids encoding a xylE marker gene (C. Winstanley, J. A. W. Morgan, R. W. Pickup, J. G. Jones, and J. R. Saunders, Appl. Environ. Microbiol. 55:771-777, 1989). A series of direct membrane filter methods have been developed which facilitate the detection of bacterial cells harboring the xylE gene, its product, catechol 2,3-dioxygenase, and catechol 2,3-dioxygenase enzyme activity directly from water samples. These methods enable detection of recombinant populations at concentrations as low as 10(3) to 10(4) cells ml of lake water-1. Direct detection facilitates ecological studies of a range of bacterial strains containing the marker system in aquatic environments. The fate of a recombinant pseudomonad population in lake water was assessed by a combination of colony-forming ability, direct counts, and direct detection of the xylE gene and phenotypic expression of its product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Morita R. Y. Protein Patterns of Growing and Starved Cells of a Marine Vibrio sp. Appl Environ Microbiol. 1983 Jun;45(6):1748–1752. doi: 10.1128/aem.45.6.1748-1752.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentjen S. A., Fredrickson J. K., Van Voris P., Li S. W. Intact soil-core microcosms for evaluating the fate and ecological impact of the release of genetically engineered microorganisms. Appl Environ Microbiol. 1989 Jan;55(1):198–202. doi: 10.1128/aem.55.1.198-202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. D., Fiksdal L. Rapid detection of total and fecal coliforms in water by enzymatic hydrolysis of 4-methylumbelliferone-beta-D-galactoside. Appl Environ Microbiol. 1988 Aug;54(8):2118–2122. doi: 10.1128/aem.54.8.2118-2122.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fredrickson J. K., Bezdicek D. F., Brockman F. J., Li S. W. Enumeration of Tn5 mutant bacteria in soil by using a most- probable-number-DNA hybridization procedure and antibiotic resistance. Appl Environ Microbiol. 1988 Feb;54(2):446–453. doi: 10.1128/aem.54.2.446-453.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff K. A. Rapid and simple method for double staining of bacteria with 4',6-diamidino-2-phenylindole and fluorescein isothiocyanate-labeled antibodies. Appl Environ Microbiol. 1988 Dec;54(12):2949–2952. doi: 10.1128/aem.54.12.2949-2952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howgrave-Graham Alan R., Steyn Pieter L. Application of the Fluorescent-Antibody Technique for the Detection of Sphaerotilus natans in Activated Sludge. Appl Environ Microbiol. 1988 Mar;54(3):799–802. doi: 10.1128/aem.54.3.799-802.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaan A. J., Dahllöf B., Kjelleberg S. Changes in Protein Composition of Three Bacterial Isolates from Marine Waters during Short Periods of Energy and Nutrient Deprivation. Appl Environ Microbiol. 1986 Dec;52(6):1419–1421. doi: 10.1128/aem.52.6.1419-1421.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. G., Simon B. M. An investigation of errors in direct counts of aquatic bacteria by epifluorescence microscopy, with reference to a new method for dyeing membrane filters. J Appl Bacteriol. 1975 Dec;39(3):317–329. doi: 10.1111/j.1365-2672.1975.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Kemp H. A., Archer D. B., Morgan M. R. Enzyme-Linked Immunosorbent Assays for the Specific and Sensitive Quantification of Methanosarcina mazei and Methanobacterium bryantii. Appl Environ Microbiol. 1988 Apr;54(4):1003–1008. doi: 10.1128/aem.54.4.1003-1008.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levanony H., Bashan Y., Kahana Z. E. Enzyme-Linked Immunosorbent Assay for Specific Identification and Enumeration of Azospirillum brasilense Cd. in Cereal Roots. Appl Environ Microbiol. 1987 Feb;53(2):358–364. doi: 10.1128/aem.53.2.358-364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario A. J., Conway de Macario E. Quantitative immunologic analysis of the methanogenic flora of digestors reveals a considerable diversity. Appl Environ Microbiol. 1988 Jan;54(1):79–86. doi: 10.1128/aem.54.1.79-86.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Muyzer G., de Bruyn A. C., Schmedding D. J., Bos P., Westbroek P., Kuenen G. J. A Combined Immunofluorescence-DNA-Fluorescence Staining Technique for Enumeration of Thiobacillus ferrooxidans in a Population of Acidophilic Bacteria. Appl Environ Microbiol. 1987 Apr;53(4):660–664. doi: 10.1128/aem.53.4.660-664.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai C., Hori K., Kagamiyama H., Nakazawa T., Nozaki M. Purification, subunit structure, and partial amino acid sequence of metapyrocatechase. J Biol Chem. 1983 Mar 10;258(5):2916–2922. [PubMed] [Google Scholar]
- Nakai C., Kagamiyama H., Nozaki M., Nakazawa T., Inouye S., Ebina Y., Nakazawa A. Complete nucleotide sequence of the metapyrocatechase gene on the TOI plasmid of Pseudomonas putida mt-2. J Biol Chem. 1983 Mar 10;258(5):2923–2928. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Shields M. S., Tedford E. T., Breen A., Hooper S. W., Sirotkin K. M., Davis J. W. Application of DNA-DNA colony hybridization to the detection of catabolic genotypes in environmental samples. Appl Environ Microbiol. 1985 May;49(5):1295–1303. doi: 10.1128/aem.49.5.1295-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A. P., Wang T. T., Greenberg D. B. Pattern recognition analysis of in vivo enzyme-substrate fluorescence velocities in microorganism detection and identification. Appl Environ Microbiol. 1986 May;51(5):969–977. doi: 10.1128/aem.51.5.969-977.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C., Morgan J. A., Pickup R. W., Jones J. G., Saunders J. R. Differential regulation of lambda pL and pR promoters by a cI repressor in a broad-host-range thermoregulated plasmid marker system. Appl Environ Microbiol. 1989 Apr;55(4):771–777. doi: 10.1128/aem.55.4.771-777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]