Abstract
MKK4 is a member of the mitogen-activated protein kinase kinase group of dual specificity protein kinases that functions as an activator of the c-Jun NH2-terminal kinase (JNK) in vitro. To examine the function of MKK4 in vivo, we investigated the effect of targeted disruption of the MKK4 gene. Crosses of heterozygous MKK4 (+/−) mice demonstrated that homozygous knockout (−/−) animals die before embryonic day 14, indicating that the MKK4 gene is required for viability. The role of MKK4 in JNK activation was examined by investigation of cultured MKK4 (+/+) and MKK4 (−/−) cells. Disruption of the MKK4 gene blocked JNK activation caused by: (i) the mitogen-activated protein kinase kinase kinase MEKK1, and (ii) treatment with anisomycin or heat shock. In contrast, JNK activation caused by other forms of environmental stress (UV-C radiation and osmotic shock) was partially inhibited in MKK4 (−/−) cells. Regulated AP-1 transcriptional activity, a target of the JNK signal transduction pathway, was also selectively blocked in MKK4 (−/−) cells. Complementation studies demonstrated that the defective AP-1 transcriptional activity was restored by transfection of MKK4 (−/−) cells with an MKK4 expression vector. These data establish that MKK4 is a JNK activator in vivo and demonstrate that MKK4 is an essential component of the JNK signal transduction pathway.
Three distinct groups of mammalian enzymes closely related to mitogen-activated protein (MAP) kinases have been identified: extracellular signal-regulated kinase, p38 MAP kinase, and c-Jun NH2-terminal kinase (JNK) (1). These MAP kinases phosphorylate distinct groups of substrates in vitro and have been implicated in the regulation of fundamental cellular processes (1, 2). These enzymes are activated by dual phosphorylation on Thr and Tyr within the motif Thr-Xaa-Tyr located in kinase subdomain VIII (3). This phosphorylation is mediated by a protein kinase cascade that is composed of a MAP kinase kinase kinase that phosphorylates and activates one or more MAP kinase kinases that, in turn, phosphorylates and activates each MAP kinase. The signaling pathways that lead to MAP kinase activation are highly conserved and have been identified in many organisms, including yeast, worms, flies, and humans (3).
The JNK group of MAP kinases includes at least 10 members (4) that are activated in response to a wide array of cellular stresses, including osmotic shock, heat shock, lipopolysaccharide, protein synthesis inhibitors, UV irradiation, and certain cytokines (4–8). JNK phosphorylates two residues (Ser-63 and Ser-73) on the NH2-terminal activation domain of c-Jun (5, 9, 10). Similarly, JNK phosphorylate two sites (Thr-69 and Thr-71) on the NH2 terminal activation domain of the transcription factor ATF2 (11–13). The phosphorylation of these transcription factors by JNK leads to increased transcriptional activity (1). The c-Jun and ATF2 proteins are members of the bZIP group that function as components of AP-1 and AP-1-like transcription factors. Thus, the activation of c-Jun and ATF2 by JNK has been proposed to account, in part, for the regulation of AP-1 transcriptional activity (1, 2).
The JNKs are activated by phosphorylation on Thr and Tyr (5) by the dual specificity protein kinase MKK4 (also known as SEK1/JNKK), a member of the MAP kinase kinase group (14–16). MKK4 is, in turn, activated by dual phosphorylation on Ser and Thr within kinase subdomain VIII by MAP kinase kinase kinases, including MEKK1 (17, 18). The ability of MKK4 to activate JNK is not shared by any of the other MAP kinases kinases (MKK1, MKK2, MKK3, MKK5, and MKK6) that have been molecularly cloned (1). Furthermore, dominant-negative MKK4 acts as a specific inhibitor of the JNK signal transduction pathway (19–22). These data indicate a central role for the MAP kinase kinase MKK4 in the JNK signal transduction pathway (1). However, direct evidence for a role of MKK4 in the regulation of JNK activity in vivo has not been reported.
To examine the physiological role of MKK4, we have investigated the effect of disruption of the MKK4 gene. Mice with a homozygous null allele for MKK4 are not viable, indicating that MKK4 is an essential gene. Loss of MKK4 causes embryonic death. Studies of wild-type (+/+) and MKK4 (−/−) cells in culture demonstrate that MKK4 is required for the normal regulation of the JNK signal transduction pathway. MKK4 (−/−) cells exhibit defects in both JNK activity and AP-1 transcriptional activity. Together, these data establish that MKK4 functions as an activator of the JNK protein kinase in vivo and demonstrate that MKK4 is an essential component of the JNK signal transduction pathway.
MATERIALS AND METHODS
Embryonic Stem (ES) Cell Culture.
W9.5 embryonic stem cells (kindly provided by C. L. Stewart, Roche Institute of Molecular Biology, Nutley, NJ) were grown in gelatinized tissue culture plates in DMEM supplemented with 15% heat-inactivated fetal bovine serum, 2 mM glutamine, 0.1 mM 2-mercaptoethanol, 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 1,000 units/ml of leukemia inhibitory factor (GIBCO/BRL) at 37°C in a humidified atmosphere of 10% CO2.
Gene Targeting and Generation of MKK4 (−/−) ES Cells.
The PGKneo cassette and herpes simplex virus (HSV)-thymidine kinase gene vector pBSNTK2 was constructed using pBluescript SK (Stratagene) as the plasmid backbone. A 1.6-kb EcoRI–BglII fragment carrying the PGKneo cassette (kindly provided by D. Y. Loh, then at Washington University School of Medicine, St. Louis) was inserted into the BamHI and EcoRI sites of pBluescript SK (Stratagene). A 3.4-kb fragment containing two copies of the herpes simplex virus type-1 thymidine kinase gene (23) was inserted into the XhoI site (Fig. 1).
Figure 1.
Strategy for the targeted disruption of the MKK4 gene. The pBSNTK2 vector, the MKK4 targeting vector, the genomic region at the MKK4 locus, and the predicted structure of the mutated MKK4 gene. Restriction enzyme sites are indicated (B, BamHI; R, EcoRI; N, NotI; X, XhoI; Ap, ApaI; Sp, SpeI; Xb, XbaI). The two hatched boxes are MKK4 exons corresponding to the subdomain VII and VIII (amino acid residues 211–268). SIAKT is the sequence (single-letter code) that includes the dual phosphorylation sites that are required for MKK4 activation.
Genomic DNA clones corresponding to the MKK4 locus were cloned from a λ FixII phage library prepared from mouse strain 129/Sv (Stratagene). The targeting vector was constructed by inserting a 5.5-kb SpeI–XbaI fragment obtained from the 5′ end of the MKK4 genomic clone into the XbaI site of pBSNTK2. A 927-bp ApaI fragment derived from the 3′ end of the MKK4 genomic clone was inserted into the vector at the XhoI site using XhoI linkers. The resulting plasmid was linearized with NotI and electroporated into W9.5 ES cells. Genomic DNA from transfectants resistant to G418 (200 μg/ml) (GIBCO/BRL) and gancyclovir (2 μM) (Syntex, Palo Alto, CA) were characterized by Southern blot analysis. Homozygous mutant ES cell clones were obtained by selection of the heterozygous ES cells in 1.5 mg/ml G418 (24).
Hybridization Analysis.
Genomic DNA isolated from mouse tails and ES cells was digested with EcoRI (or other restriction enzymes) and examined by Southern blot analysis. The probe was prepared from the 3′ region of the MKK4 genomic sequence (Fig. 1). This probe hybridizes to a 6.0-kb fragment (endogenous MKK4 gene) and to a 2.5-kb fragment (disrupted allele).
Northern blot analysis was performed using total RNA (10 μg) prepared from ES cells using the TRIzol reagent (GIBCO/BRL). The blots were hybridized to a radiolabeled probe corresponding to nucleotides 772-1048 of the murine MKK4 cDNA (15).
Reverse Transcriptase–PCR.
Total RNA was prepared from ES cells using the TRIzol reagent (GIBCO/BRL). The first-strand cDNA was obtained by reverse transcription. PCR was performed according to the manufacturer’s protocol (Sangon, Scarborough, Ontario, Canada). Hypoxanthine–guanine phosphoribosyltransferase was amplified by PCR (25). The amplimers used for PCR of MKK4 were: 5′-TGGACAGCTTGTGGACTCTATTGC-3′ and 5′-CTGGCATCTGATCCAGGATTTTAC-3′. The 413-bp DNA product corresponds to nucleotides 772–1184 of the mouse MKK4 cDNA (15).
Western Blot Analysis.
Immunoblot analysis of cell lysates was performed by probing with monoclonal antibodies to MKK4 and JNK (PharMingen). Immunecomplexes were detected by enhanced chemiluminescence (Amersham).
Protein Kinase Activity.
JNK and p38 MAP kinase activity in cell lysates was measured by immunecomplex protein kinase assays using the substrates glutathione S-transferase (GST)-cJun and GST-ATF2, respectively (8).
AP-1-Dependent Reporter Gene Expression.
Transfection assays were performed using ES cells and the Lipofectamine method (GIBCO/BRL). The cells were cotransfected with 0.5 μg of pTRE-Luciferase (26) and 0.5 μg of pCH110 (Pharmacia-LKB) together with 0.5 μg of pCMV5, 0.5 μg of pCMV-Ras61L (27), or 0.5 μg of pCMV-MEKK1 (20). In some experiments the cells also were transfected with 0.5 μg of pCDNA3 (Invitrogen), pCDNA3-MKK4 (14), or pCDNA3-Flag-JNK1 (5). Experiments using GAL4 fusion proteins were performed using 0.5 μg of pGAL4-Jun (residues 1–223) and 0.5 μg of the reporter plasmid pG5E1bLuc (4). The cells were harvested 36 h posttransfection. The β-galactosidase and luciferase activities in the cell lysates were measured as described (20).
RESULTS
Targeted Disruption of the Mouse MKK4 Gene.
We used the positive-negative selection strategy (28) to inactivate the mouse MKK4 gene. Initially, the plasmid pBSNTK2 was constructed using PGKneo and two copies of the herpes simplex virus type-1 thymidine kinase gene flanked by unique cloning sites (Fig. 1). A 5.5-kb SpeI-XbaI fragment obtained from the 5′ end of a MKK4 genomic clone was inserted in the XbaI site of pBSNTK2. A 927-bp ApaI fragment derived from the 3′ end of the MKK4 genomic clone also was inserted into the vector (Fig. 1). A targeted recombination event involving this vector would replace an internal 3.5-kb XbaI–ApaI genomic fragment with the neo gene cassette. The deleted region encompasses two exons encoding amino acids 211–268 of the murine MKK4 sequence (15). This region includes the sequence Ser-Ile-Ala-Lys-Thr that surrounds the dual phosphorylation sites (Ser and Thr) that are required for MKK4 activation (14–17). Mutation of these phosphorylation sites inhibits MKK4 protein kinase activity (14). We therefore anticipated that the predicted targeted disruption of the MKK4 gene should result in a null allele.
The linearized targeting construct was transfected into W9.5 ES cells. Analysis of 73 independent G418 and gancyclovir-resistant clones by Southern blotting identified 12 clones with a pattern consistent with the expected homologous recombination. An example of one clone is shown in Fig. 2. The frequency of homologous recombinants among the G418 and gancyclovir-resistant clones was 16.4%. Seven targeted ES clones were injected into C57BL/6 (B6) blastocysts, and two (clones 7 and 9) resulted in chimeric mice that transmitted the disrupted MKK4 allele through the germ line. The heterozygous mice appeared to be healthy; they were fertile and of normal size. Progeny from intercrosses of these heterozygous mice were analyzed for genotype at 3 weeks after birth (Table 1). The ratio of wild-type to heterozygous littermates was 1.0:1.6. The birth of homozygous MKK4 (−/−) mice was not obtained from this cross. We therefore examined the fetuses between embryonic day E14.5 and E15.5. No homozygous MKK4 (−/−) fetuses were found and the ratio of MKK4 (+/+) to MKK4 (+/−) fetuses was 1.0:1.8 (Table 1). These data demonstrate that mice homozygous for the disrupted MKK4 allele (−/−) die before E14. MKK4 therefore is required for normal early embryonic development.
Figure 2.
Identification of ES cell clones with targeted disruption of the MKK4 gene. (A) Genomic DNA prepared from ES cell clones indicates the presence of all three expected genotypes. EcoRI restricted DNA was probed with a radiolabeled fragment of the MKK4 gene (Fig. 1). The upper band (6 kb) corresponds to the wild-type allele, and the lower band (2.5 kb) corresponds to the mutant allele. (B) Northern blot analysis of total RNA (10 μg) isolated from wild-type and homozygous knockout ES cells. Blots were probed with a random-primed 32P-labeled mouse MKK4 cDNA probe. The blots also were probed for β-actin mRNA as an internal control. (C) Reverse transcription-PCR analysis of MKK4 mRNA expressed by ES cells. RNA isolated from ES cells was amplifed with primers specific for MKK4 and hypoxanthine–guanine phosphoribosyltransferase mRNA.
Table 1.
Genotypes of the progeny obtained by crossing heterozygous MKK4 (+/−) mice
| Age* | No. litters | No. pups | No. of each genotype†
|
||
|---|---|---|---|---|---|
| +/+ | +/− | −/− | |||
| E14.5 | 1 | 6 | 3 | 3 | 0 |
| E15.5 | 3 | 14 | 4 | 10 | 0 |
| Day 21 | 7 | 34 | 13 | 21 | 0 |
| Total | 11 | 54 | 20 (37%)‡ | 34 (63%)‡ | 0 |
E14.5 and E15.5 represent day 14.5 and 15.5 of gestation; Day 21 represents 21st day after birth.
+/+, Wild type; +/−, heterozygous; −/−, homozygous for the MKK4 allele.
The percentage of pups with each genotype.
To obtain homozygous MKK4 (−/−) ES cells, one heterozygous clone (no. 7) was expanded and exposed to 1.5 mg/ml of G418. After selection for 2 weeks, three clones with disruption of both copies of the MKK4 gene were generated. The homozygous genotype was determined by Southern analysis of genomic DNA (Fig. 2A). Northern blot analysis of total RNA isolated from the ES cells demonstrated that the homozygous MKK4 (−/−) cells did not express a detectable level of MKK4 mRNA (Fig. 2B). Analysis of MKK4 mRNA expression by reverse transcription-PCR confirmed this conclusion (Fig. 2C).
The expression of the MKK4 protein kinase was examined by Western blot analysis of lysates prepared from the ES cells. Blots probed with a monoclonal antibody detected MKK4 in wild-type (+/+) cells (Fig. 3A). A lower level of MKK4 was found in heterozygous (+/−) cells. In contrast, no MKK4 was detected in homozygous knockout (−/−) cells (Fig. 3A). Control experiments were performed by probing the blots with a monoclonal antibody to JNK. This antibody detects both 46-kDa and 55-kDa JNK isoforms. A similar level of JNK was detected in cells with each MKK4 genotype (Fig. 3B).
Figure 3.
Expression of MKK4 and JNK in ES cell clones. Lysates (30 μg) prepared from wild-type (+/+), heterozygous (+/−), and homozygous knockout (−/−) MKK4 cells were examined by Western blot analysis using antibodies to MKK4 (A) and JNK (B). The cells were treated without (−) and with (+) UV-C radiation (80 J/m2) and harvested after incubation at 37°C for 1 h. Open arrows indicate MKK4 (45 kDa) and JNK (46 kDa and 55 kDa). Closed arrows indicate the migration of molecular mass standards (kDa).
Together, these data demonstrated that the disrupted MKK4 gene was a null allele. The homozygous MKK4 (−/−) cells were defective in MKK4 expression, but not in the expression of the MKK4 substrate JNK.
MKK4 Gene Disruption Causes Defects in JNK Activity.
MKK4 is an activator of the JNK group of MAP kinases (14–16). We therefore examined the effect of MKK4 gene disruption on JNK protein kinase activity. Exposure of wild-type cells to several forms of environmental stress caused a marked increase in JNK protein kinase activity (Fig. 4). A similar (although reduced) increase in JNK activity was detected in experiments using heterozygous MKK4 (+/−) cells. In contrast, homozygous knockout MKK4 (−/−) cells were defective in the activation of JNK by these stimuli (Fig. 4). Heat shock and anisomycin treatment did not activate JNK. However, osmotic shock (treatment with sorbitol) or exposure to UV-C radiation did cause JNK activation in the homozygous MKK4 (−/−) cells, although the extent of JNK activation was lower than that detected in wild-type cells (Fig. 4).
Figure 4.
Effect of MKK4 gene disruption on JNK activation. Wild-type (+/+), heterozygous (+/−), and homozygous knockout (−/−) MKK4 cells were untreated (lane 1) or treated with (lane 2) UV-C (80 J/m2), (lane 3) osmotic shock (300 mM sorbitol), (lane 4) heat shock (42°C, 15 min.), or (lane 5) anisomycin (5 μg/ml). The cells were harvested after incubation at 37°C for 1 h. JNK activity was measured using an immunecomplex kinase assay with the substrate GST-cJun. The radioactivity incorporated into GST-cJun was quantitated after SDS/PAGE by Phosphorimager analysis.
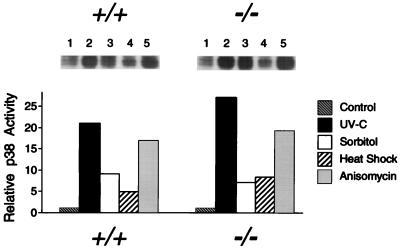
The difference in JNK regulation observed for wild-type (+/+) and MKK4 (−/−) cells might have been caused by a specific defect present in the JNK signaling pathway of MKK4 (−/−) cells. Alternatively, the differences observed may be caused by a more general defect in the response of the MKK4 (−/−) cells to environmental stress. We therefore examined the activation of p38 MAP kinase, which is also activated by exposure of cells to environmental stress (8). No marked differences were found in the activation of p38 MAP kinase in experiments using wild-type (+/+) and MKK4 (−/−) cells (Fig. 5). These data indicated that the MKK4 (−/−) cells were not defective in their ability to respond to environmental stimulation. Instead, these data demonstrated that the MKK4 (−/−) cells have a specific defect in the JNK signal transduction pathway.
Figure 5.
Effect of MKK4 gene disruption on p38 MAP kinase activation. Wild-type (+/+) and homozygous knockout (−/−) MKK4 cells were untreated (lane 1) or treated with (lane 2) UV-C (80 J/m2), (lane 3) osmotic shock (300 mM sorbitol), (lane 4) heat shock (42°C, 15 min), or (lane 5) anisomycin (5 μg/ml). The cells were harvested after incubation at 37°C for 1 h. p38 MAP kinase activity was measured using an immunecomplex kinase assay with the substrate GST-ATF2. The radioactivity incorporated into GST-ATF2 was quantitated after SDS/PAGE by PhosphorImager analysis.
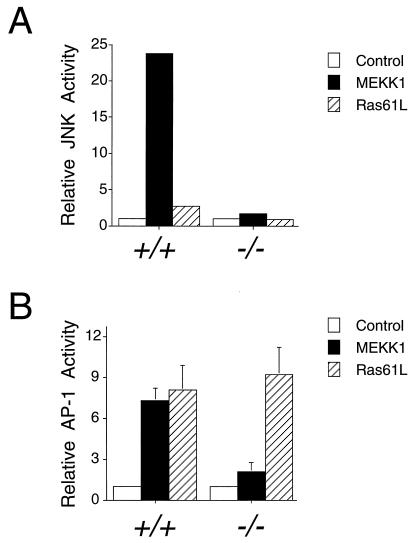
The defect in JNK activation in MKK4 (−/−) cells was further examined by investigating the regulation of JNK by an upstream component of the JNK signal transduction pathway. The MAP kinase kinase kinase MEKK1 is a powerful activator of the JNK pathway that phosphorylates MKK4 (14–18). To test the effect of MEKK1 on JNK activity in wild-type and MKK4-deficient cells, we performed cotransfection assays using MEKK1 and epitope-tagged JNK1. Immunecomplex kinase assays demonstrated that MEKK1 caused JNK activation in experiments using wild-type cells (Fig. 6A). In contrast, MEKK1 did not activate JNK in homozygous knockout MKK4 (−/−) cells (Fig. 6A). These data demonstrated that MKK4 was required for the normal regulation of JNK protein kinase activity.
Figure 6.
MKK4 is required for MEKK1-induced JNK activation and AP-1 transcriptional activity. (A) Wild-type (+/+) and homozygous knockout (−/−) MKK4 cells were cotransfected with pCDNA3-Flag-JNK1 together with pCMV5, pCMV5-MEKK1, or pCMV-Ras61L. The epitope-tagged JNK1 was immunoprecipitated with the M2 monoclonal antibody. Protein kinase activity in the immunecomplexes was measured using the substrate GST-cJun. The radioactivity incorporated into GST-cJun was quantitated after SDS/PAGE by Phosphorimager analysis. (B) Wild-type (+/+) and homozygous knockout (−/−) MKK4 cells were cotransfected with the TRE-luciferase reporter plasmid together with activated Ras or MEKK1. Transfection efficiency was monitored using the β-galactosidase expression vector pCH110. The relative AP-1-dependent reporter gene expression is presented as the activity ratio of luciferase/β-galactosidase. Data are the mean ± SD (n = 3).
MKK4 Gene Disruption Causes Defects in AP-1 Transcriptional Activity.
The JNK signal transduction pathway has been implicated in AP-1 dependent gene expression (1). To test the role of MKK4, we performed cotransfection assays with a TRE-luciferase reporter plasmid in experiments using wild-type and MKK4-deficient cells. Control experiments demonstrated that Ras-activated AP-1 dependent reporter gene expression was similar in wild-type (+/+) and MKK4 (−/−) cells (Fig. 6B). In contrast, the MEKK1-stimulated reporter gene expression observed in wild-type (+/+) cells was absent in MKK4 (−/−) cells (Fig. 6). These data indicated that MKK4 gene disruption caused a selective defect in AP-1 transcriptional activity.
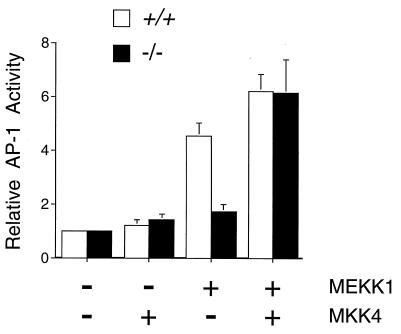
To confirm that the defect in AP-1 transcriptional activity observed in MKK4 (−/−) cells was due to loss of MKK4 expression, we performed complementation analysis (Fig. 7). Transfection with an MKK4 expression vector, but not with an empty expression vector, restored MEKK1-stimulated AP-1 dependent gene expression in the homozygous knockout MKK4 (−/−) cells (Fig. 7). These data demonstrated that MKK4 was required for the normal regulation of AP-1 dependent gene expression.
Figure 7.
Complementation analysis of MKK4 gene disruption. Wild-type (+/+) and homozygous knockout (−/−) MKK4 cells were cotransfected with the TRE-luciferase reporter plasmid together with MKK4 or MEKK1 expression vectors, as indicated. Transfection efficiency was monitored using the β-galactosidase expression vector pCH110. The relative AP-1-dependent reporter gene expression is presented as the activity ratio of luciferase/β-galactosidase. Data are the mean ± SD (n = 3).
The AP-1 transcription factor is composed of dimeric complexes formed by members of the Jun and Fos families. To biochemically dissect the role of c-Jun, we examined the transcriptional activity of the c-Jun activation domain (residues 1–223) fused to the GAL4 DNA binding domain using a luciferase reporter plasmid. Ras and MEKK1 caused increased GAL4-Jun dependent luciferase activity (18). This activation of GAL4-Jun was suppressed in experiments using MKK4 (−/−) cells (data not shown). Similarly, the transcriptional activity of GAL4-Jun induced by Ras and MEKK1 was suppressed by mutation of the JNK phosphorylation sites (Ser-63 and Ser-73) on c-Jun (data not shown). These data demonstrate that MKK4 and the JNK signaling pathway contribute to the activation of c-Jun by Ras and MEKK1. However, MKK4 was not required for the increased AP-1 dependent gene expression caused by Ras (Fig. 6). The effect of Ras on AP-1 dependent gene expression may therefore be mediated, in part, by a pathway that is independent of c-Jun regulation (e.g. the induction of c-Fos).
DISCUSSION
Targeted Disruption of the MKK4 Gene Causes Embryonic Lethality in Mice.
MKK4 is an essential gene that is required for viability in mice. Homozygous disruption of the MKK4 gene causes death before embryonic day 14 (Table 1). The requirement of MKK4 for viability suggests that the JNK signal transduction pathway is necessary for normal embryonic development. However, the precise role of MKK4 is unclear. Recent studies of Drosophila suggest that JNK signaling regulates morphogenetic cell movements during embryonic development. Loss-of-function alleles of the Drosophila homologs of both MKK4 (hep) and JNK (bsk) cause similar defects in embryonic development (29–31). Genetic analysis of hep and bsk in Drosophila demonstrates that these genes are required for specific changes in cell morphology and migration, but the molecular mechanism of action of these genes remains to be established (29–31). These studies of Drosophila development represent a useful framework for the study of the JNK signaling pathway in mammalian cells. Indeed, it is possible that, like Drosophila, the mammalian JNK pathway is required for specific stages of embryonic morphogenesis. Current studies are designed to test this hypothesis.
MKK4 Functions as a JNK Activator in Vivo.
In vitro studies demonstrate that MKK4 phosphorylates and activates JNK (14–16). Indirect evidence obtained from transfection studies indicate that MKK4 may activate JNK in vivo (14–16). However, a direct test of this hypothesis has not been reported. The results of the present study establish that MKK4 does activate JNK in vivo. We show that MKK4 is required for the activation of JNK caused by exposure of cells to anisomycin and heat shock (Fig. 4). Furthermore, the MAP kinase kinase kinase MEKK1, a powerful activator of the JNK pathway in wild-type cells (17, 18), does not increase JNK activity in homozygous knockout MKK4 (−/−) cells (Fig. 5). Together, these data demonstrate that MKK4 is an essential component of the JNK signal transduction pathway.
Through our studies, it is established that MKK4 is required for JNK activation caused by MEKK1 and exposure of cells to anisomycin or heat shock (Figs. 4 and 5). However, MKK4 was not found to be essential for JNK activation caused by other stimuli. For example, exposure of cells to osmotic shock and UV radiation caused JNK activation in homozygous knockout MKK4 (−/−) cells, although the extent of JNK activation in MKK4 (−/−) cells was less than that detected in wild-type cells (Fig. 4). Thus, MKK4 may contribute to JNK activation caused by osmotic shock and UV radiation, but MKK4 may not be the only activator of JNK in these cells. None of the other MAP kinase kinase isoforms that have been characterized (MKK1, MKK2, MKK3, MKK5, and MKK6) are able to activate JNK (1), indicating the possible existence of at least one novel JNK activator (MKK7) that remains to be molecularly cloned. Different stimuli therefore may activate one or both of these JNK activation pathways in vivo. Loss of MKK4 therefore causes a selective defect in the response of the JNK signal transduction pathway to specific environmental stimuli. Comparison of wild-type (+/+) and MKK4 (−/−) cells indicates that MKK4 is a major component of the JNK signal transduction pathway in ES cells (Fig. 4).
Biochemical studies demonstrate that MKK4 can phosphorylate and activate both JNK and p38 MAP kinase (14, 16). However, the homozygous knockout MKK4 (−/−) cells were found to be defective in JNK activation (Fig. 4), but not in the activation of p38 MAP kinase (Fig. 5). These data suggest that MKK4 may function as a specific activator of JNK in vivo. Alternatively, it is possible that the effect of MKK4 gene disruption is partially complemented by the p38 MAP kinase activators MKK3 (14) and MKK6 (32–35). Further studies are required to establish whether MKK4 plays a role in the p38 MAP kinase signal transduction pathway in vivo.
AP-1 Transcriptional Activity Is Regulated by the MKK4 Signaling Pathway.
Previous studies have implicated the transcription factor AP-1 as an important target of the JNK signal transduction pathway (1). The effect of JNK probably is mediated by multiple mechanisms. For example, JNK activates c-Jun and ATF2 by phosphorylating the NH2 terminal activation domains of these transcription factors (1). In addition, JNK increases c-Jun expression through AP-1 sites located in the c-Jun promoter (36). The expression of c-Jun also is increased by inhibition of the ubiquitin-mediated degradation of c-Jun (37). Finally, JNK increases c-Fos expression, in part, through the serum-response element located in the c-Fos promoter (20, 38, 39). Together, these data indicate that the AP-1 transcription factor is a target of the JNK signaling pathway (1). However, although there is substantial biochemical evidence supporting a role for the JNK pathway in the regulation of AP-1, a direct test of this hypothesis has not been previously obtained.
We examined AP-1 transcriptional activity in wild-type (+/+) and homozygous knockout MKK4 (−/−) cells using an AP-1-dependent reporter gene. MEKK1 caused a marked increase in both JNK activation and AP-1-dependent reporter gene expression in wild-type cells (Fig. 6). In contrast, MEKK1 failed to activate JNK or reporter gene expression in homozygous knockout MKK4 (−/−) cells (Fig. 6). This defect in the MKK4 (−/−) cells was complemented by expression of MKK4 (Fig. 7). Together, these data strongly support the hypothesis that the JNK pathway regulates AP-1 transcriptional activity in vivo.
The MKK4 (−/−) cells exhibit a marked defect in AP-1 transcriptional activity (Fig. 6). However, these cells are not completely defective in AP-1 transcriptional regulation. For example, AP-1 transcriptional activity is increased by Ras in both wild-type and MKK4 (−/−) cells (Fig. 6). This effect of Ras may be mediated by the extracellular signal-regulated kinase signal transduction pathway (1), which can activate AP-1 (40) through increased c-Fos expression (41). As Ras is a poor activator of JNK (5), it is likely that only a small component of the response of wild-type cells to activated Ras is mediated by the JNK pathway (Fig. 6). The loss of MKK4 therefore causes a selective block in the regulation of AP-1 transcriptional activity.
Conclusions.
The results of this study establish that MKK4 is a JNK activator in vivo and demonstrate that MKK4 is an essential component of the JNK signal transduction pathway. Disruption of the MKK4 gene causes a selective block in the JNK signaling pathway. Targets of the JNK include the AP-1 transcription factor.
Acknowledgments
We thank D. Y. Loh and C. L. Stewart for providing reagents, T. Barrett for DNA sequencing, L. Evangelisti, C. Hughes, and D. Butkis for their excellent technical assistance, and K. Gemme for administrative assistance. This study was supported by Grant CA65861 (R.J.D.) from the National Institutes of Health and by the Howard Hughes Medical Institute. C.T. was supported by an Association pour la Recherche contre le Cancer fellowship. R.J.D. and R.A.F. are Investigators, and D.Y. and H.-T.L. are Associates of the Howard Hughes Medical Institute.
ABBREVIATIONS
- JNK
c-Jun NH2-terminal kinase
- MAP
mitogen-activated protein
- ES
embryonic stem
- GST
glutathione S-transferase
References
- 1.Whitmarsh A J, Davis R J. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 2.Karin M. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 3.Davis R J. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Derijard B, Davis R J. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 5.Dérijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 6.Sluss H K, Barrett T, Dérijard B, Davis R J. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 8.Raingeaud J, Gupta S, Rogers J, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 9.Smeal T, Binetruy B, Mercola D A, Birrer M, Karin M. Nature (London) 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- 10.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Nature (London) 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Campbell D, Dérijard B, Davis R J. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 12.Livingstone C, Patel G, Jones N. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dérijard B, Raingeaud J, Barrett T, Wu I-H, Han J, Ulevitch R J, Davis R J. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Nature (London) 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 16.Lin A, Minden A, Martinetto H, Claret F-X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 17.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Nature (London) 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 18.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 19.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 20.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 21.Zanke B W, Boudreau K, Rubie E, Winnett E, Tibbles L A, Zon L, Kyriakis J, Liu F-F, Woodgett J R. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 22.Verheij M, Bose R, Lin X H, Yao B, Jarvis D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 23.McKnight S L. Nucleic Acids Res. 1980;8:5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortensen R M, Conner D A, Chao S, Geisterfer-Lowrance A A, Seidman J G. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson G G, Kronert W A, Bernstein S I, Chapman V M, Smith K D. J Biol Chem. 1988;263:9079–9082. [PubMed] [Google Scholar]
- 26.Rincon M, Flavell R A. EMBO J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wartmann M, Davis R J. J Biol Chem. 1994;269:6695–6701. [PubMed] [Google Scholar]
- 28.Mansour S L, Thomas K R, Capecchi M R. Nature (London) 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 29.Glise B, Bourbon H, Noselli S. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 30.Sluss H K, Han Z, Barrett T, Davis R J, Ip T. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 31.Riesgo-Escovar J R, Jenni M, Fritz A, Hafen E. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- 32.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han J, Lee J-D, Jiang Y, Li Z, Feng L, Ulevitch R J. J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 34.Stein B, Brady H, Yang M X, Young D B, Barbosa M S. J Biol Chem. 1996;271:11427–11433. doi: 10.1074/jbc.271.19.11427. [DOI] [PubMed] [Google Scholar]
- 35.Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 36.Angel P, Hattori K, Smeal T, Karin M. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs S Y, Dolan L, Davis R J, Ronai Z. Oncogene. 1996;13:1531–1535. [PubMed] [Google Scholar]
- 38.Cavigelli M, Dolfi F, Claret F-X, Karin M. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gille H, Strahl T, Shaw P E. Curr Biol. 1995;5:1191–1200. doi: 10.1016/s0960-9822(95)00235-1. [DOI] [PubMed] [Google Scholar]
- 40.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 41.Marais R, Wynne J, Treisman R. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]