Summary
Our understanding of neuronal migration has been advanced by multidisciplinary approaches. At the cellular level, tangential and radial modes of neuronal migration contribute to different populations of neurons and have differential dependence on glial cells. At the molecular level, extracellular guidance cues have been identified and intracellular signal transduction pathways are beginning to be revealed. Interestingly, mechanisms guiding axon projection and neuronal migration appear to be conserved with those for chemotactic leukocytes.
Introduction
The nervous system is populated with natural migrants; the majority of, if not all, neuronal precursors have to migrate from their place of origin to sites of final residence and functioning. The possibility of neuronal migration was raised by Kolliker, His, Magini, Ramon y Cajal, and Vignal in the late part of the 19th century(1) and is now well established as an important process in neural development.(2,3) The peripheral nervous system (PNS) is largely derived from neural crest cells migrating out of the dorsal part of the neural tube. In the central nervous system (CNS), neuronal precursor cells in the embryonic ventricular zone can move to other layers in the same brain region by radial migration or to other brain regions by tangential migration.(2) Neuronal migration is not limited to embryonic development because it is also found in neonatal and adult brains. Studies of neuronal migration are important not only for revealing basic mechanisms of neural development but also for understanding the etiology of human diseases caused by abnormal neuronal migration.
Two modes of neuronal migration
Initial categorizations of radial migration and tangential migration were based on the relative directions taken by migrating neuronal precursor cells. Radial migration is defined by neuronal migration in a direction perpendicular to the surface of the brain, whereas tangential migration describes neurons migrating in a direction parallel to the surface of the brain. During radial migration, the precursors of pyramidal neurons, the major projection neurons of the cerebral cortex, are thought to move from the ventricular zone to the pia along the fibers of radial glial cells (Fig. 1).(2,3)The outwardly migrating neurons form the cortical plate, which separates the preplate. This primitive lamination of the neocortex proceeds in an inside-out pattern in that new cells take more superficial positions whereas old cells are positioned in deeper layers of the cortical plates.(4) During cerebellar development, two different neuronal precursors employ radial migration for their final destination. In the embryonic cerebellum, Purkinje cells, the principal output neurons, migrate along radial glial fibers towards the surface from the neuroepithelium of cerebellar primordium.(2) In the postnatal cerebellum of rodents, cells in the external germinal layer (EGL) migrate inwards along the fibers of Bergmann gliatoform theinternalgranularlayer (IGL)(Fig. 1).(2)
Figure 1.
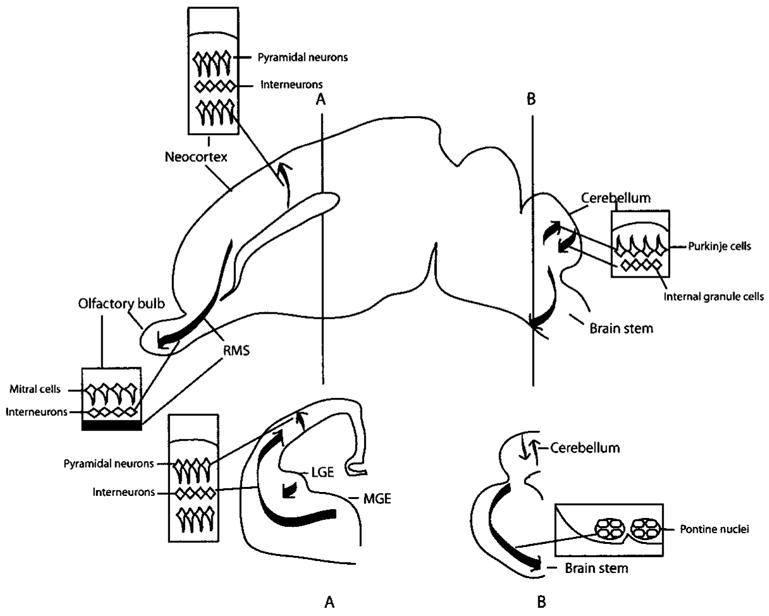
Two modes of neuronal migration in the CNS. Radial migration is involved in the development of pyramidal cells in the cortex and cerebellar granule cells, whereas tangential migration is important for the development of interneurons in the cortex and olfactory bulb, and pontine nuclei of the brain stem. A, B: Coronal sections at the levels of A and B. Abbreviations: RMS, rostral migratory stream; LGE and MGE, lateral and medial ganglionic eminences.
Although tangential migration was observed in the 1960s,(5) its importance is better appreciated in the late 1980s and 1990s (for recent reviews, see Refs. 6, 7). Tangential migration does not require glial fibers and is a major migrating mode for cells originating in the basal telencephalon (or the subpallium), known as the ganglionic eminences (GE). Significant proportions of interneurons such as the GABAergic neurons in the telencephalon are derived from these tangentially migrating neuronal precursor cells (Fig. 1). Ganglionic eminences are small masses of developing cells in the wall of the lateral ventricles of the basal telencephalon, including the lateral GE (LGE), the medial GE (MGE) and the caudal GE (CGE). Neuronal precursor cells from different GEs migrate into different regions of the brain.(6–8) For example, cells from the MGE migrate dorsally into the neocortex, whereas cells from the LGE migrate anteriorly into the olfactory bulb. Interestingly, tangential migration persists after birth especially in the subventricular zone (SVZ) of the forebrain, giving rise to more olfactory interneurons during postnatal life (Fig. 1).(9,10) In the brain stem, precursor cells in the dorsal part of developing hindbrain, the lower rhombic lip, migrate tangentially to the ventral side of the brain stem to form pontine nuclei (Fig. 1).(11)
Proteins involved in neuronal migration identified by genetic studies
Genetic studies combined with molecular cloning in mice and humans have revealed new molecules that are directly or indirectly involved in neuronal migration. The mouse mutation reeler has provided an excellent entry point for studying cortical lamination, starting with genetic and phenotypic studies.(12) reeler mutant mice have abnormal lamination of cerebral and cerebellar cortices. Histological studies of reeler mice show that the initiation of neuronal migration from the ventricular zone appears normal, and that the cell number and subtypes of neurons in the cerebral cortex also seem to be normal. However, migrating neurons in the mutant mice do not penetrate the preplate, therefore producing defects in the inside-out pattern.(13) These results indicate that the reeler mice are defective in controlling cell positioning, especially during cortical plate formation. In the cerebellum, reeler mice show abnormal lamination of the Purkinje cells.(14) Although hypotheses have been proposed to explain the phenotype of reeler mice, such as a defect in a stop signal for the migrating neurons, the precise function of the reeler gene remains controversial.
The product of the reeler gene is Reelin, a large secreted protein.(15) It is expressed primarily in the Cajal-Retzius (CR) cells in the marginal zone of the neocortex and the cerebellum.(16) Genetic and biochemical studies have demonstrated that the very low-density lipoprotein receptor (VLDLR) and the apolipoprotein E receptor-2 (ApoER2) are the receptors for Reelin.(17,18) Mice lacking either VLDLR or ApoER2 do not show abnormal phenotype, but mice lacking both genes exhibit anatomical defects almost identical to that in reeler mice.(17) The significance of additional Reelin receptors such as the cadherin-related neuronal receptors and integrin α3β1 remains unclear.(19,20)
Mice defective in the scrambler gene show a phenotype similar to that of reeler.(21) The scrambler locus was genetically mapped in the disabled-1 (Dab-1) gene on chromosome 4.(22) Targeted mutation of Dab-1 revealed a phenotype identical to that of scrambler.(23) Dab-1 is expressed in the Reelin-responsive cortical plate neurons and the Purkinje cells, with a pattern similar to those of VLDLR and ApoER2. Dab-1 is a tyrosine phosphorylated cytoplasmic protein, which can bind to the intracellular part of VLDLR and ApoER2.(17) Tyrosine phosphorylation of Dab-1 was increased by extracellular application of Reelin(24) and the level of basal tyrosine phosphorylation in reeler mice is lower than that in the wild-type mice.(24) Mutations in the phosphorylation sites of Dab-1 cause ataxia and abnormalities in cell positioning in the cerebral and cerebellar cortices.(25) There are thus strong biochemical and genetic evidence to support a pathway from the secreted protein Reelin, to the transmembrane receptors VLDLR and ApoER2, to Dab-1 phosphorylation.
There are other mouse mutants defective in cortical lamination. Mice lacking the cyclin-dependent kinase 5 (Cdk5) show defective migration in the cerebral and cerebellar cortices.(26) The defects are different from those in reeler mice, and the molecular linkage between Reelin pathway and Cdk5 is uncertain. The migrating cells could split the preplate into the marginal zone and the subplate in cdk5 mutants, but late-born neurons do not show the inside-out pattern in that they accumulate under the subplate. Cdk5 is a ubiquitously expressed serine-threonine kinase.(27) Cdk5 requires p35 and p39 proteins for activation, and mice lacking both p35 and p39 show a cortical phenotype similar to that in cdk5 mutant mice.(28) In vitro studies suggest that Cdk5 regulates cyto-skeleton dynamics through the phosphorylation of Pak or microtubule-associated proteins.(29,30)
Several human developmental disorders have been attributed to defects in neuronal migration. Lissencephaly patients show underdevelopment of the cerebral gyri probably caused by premature termination of neuronal migration.(31) The Lis-1 gene encodes a regulatory subunit of brain platelet activating factoracetylhydrolase(PAF-AH),(31)whichregulatesthemetabolism of cellular lipid PAF. PAF-AH is expressed not only in the CR cells but also in the ventricular zone of the developing neocortex.(32) PAF also regulates the migration of cerebellar granule cells.(33) It is still unclear how PAF controls neuronal migration. Zellweger syndrome is characterized by heterotopic neurons in the neocortex, and it is defective in peroxisome biogenesis.(34) Patients with mutations in the double cortex or the bicortical lissencephaly genes have two cortical plates separated by the white matter.(35) The gene product, Doublecortin, was recently identified and is a microtubule-associated protein expressed in migrating cortical cells.(35,36)
Directional guidance of neuronal migration
Progress has been made in the last few years on mechanisms guiding the direction of neuronal migration. It is now clear that migrating neurons are guided by molecular cues that also guide the projection of axons (Table 1).
Table 1.
Directional Guidance cues involved in CNS neuronal migration in vivo and in vitro
| Ligands | Receptors | Defects in CNS neuronal migration in vivo | Neuronal migration in vitro |
|---|---|---|---|
| Slits | Robo | — | |
| Netrins | DCC
Unc-5h |
||
| Semaphorins | Neuropilin
Plexin |
|
— |
| Ephrins | Eph | — |
|
Unc-5h3/RCM mutant mice showed abnormal development of cerebellum. However, it is still unclear that the defect is primarily caused by migration abnormality or other reasons.
The first molecule directly demonstrated to guide neuronal migration is the secreted protein Slit.(37) The slit gene was identified in Drosophila more than 10 years ago.(38) In 1999, three groups discovered that Slit was a repellent for axons projecting from different regions of the nervous system.(39–41) The role of Slit in neuronal migration was first discovered in the olfactory system.(37) Interneurons in the olfactory bulb are derived from precursor cells migrating in the rostral migratory stream (RMS) from the anterior subventricular zone (SVZa) in neonatal rodents.(42) Slit can repel SVZa cells, an effect that seems to require the presence of a gradient of the Slit protein.(37) Molecular studies also help to further our understanding of cellular interactions that control neuronal migration. Thus, although it was known that GABAergic neurons in the neocortex were derived from the SVZ of GE in the embryo, it was not clear why GABAergic neurons migrated out of the GE. It has now been shown that the ventricular zone in the GE contains a repulsive activity.(43) The Slit genes are expressed in the ventricular zone and Slit protein repels the GABAergic neurons.(43) Furthermore, the repulsive activity of the ventricular zone is reduced by a fragment containing only the extra-cellular part of the receptor for Slit, which blocks the interaction of Slit with the endogenous receptor.(43) These results suggest both a cellular and a molecular mechanism that may cause GABAergic neurons to migrate away from the GE. Recent genetic studies in C. elegans and Drosophila provide evidence for the involvement of Slit in cell migrations in vivo. Certain neurons in the head of C. elegans embryos migrate poster-iorly. Slit appears to repel these neurons because it is expressed in the anterior pole of the embryo and mutant embryos lacking Slit showed defects in the posterior migration of these neurons.(44) In Drosophila, the migration of the mesodermal cells that form the ventral muscles is repelled by Slit.(45)
Netrin can either be an attractant or a repellent for axons.(46,47) The basilar pontine neurons originating from the neuroepithelium of the dorsal hindbrain migrate circumferentially to the ventral midline. Netrin-1 is expressed in the ventral midline and attracts these neurons,(11) and netrin-1 mutant mice showed abnormal development of pontine nuclei.(46) It has recently been suggested that netrin in the olfactory bulb may be involved in the directional migration of SVZa cells and that the function blocking antibody to a netrin receptor, Deleted in Colorectal Cancer (DCC), blocks the directional migration of SVZa cells in RMS.(48) Netrin-1 also repels some subsets of migrating neurons in vitro. Cells from the external germinal layer of the postnatal cerebellum are repelled by netrin-1 in explant cultures.(49) Netrin has also been implicated in guiding the migration of cells from the GE into the striatum as a repellent.(50) Since both Slit and netrin can repel GE cells, it is perhaps not surprising that there was no drastic neuronal migration phenotype in mice lacking netrin.(46) It should be noted that, although Slit and netrin are repulsive to GE cells, the precise roles of these molecules in vivo are still not known. Do Slit and netrin function redundantly? Or do they act at different steps of neuronal migration from the GE?
Neurons from the GE can migrate into either the striatum or the neocortex. What controls the distinct destinations? A recent study implicates the semaphorins in this process.(51) Semaphorin 3a and 3f are expressed in the developing striatum, whereas their receptors neuropilin 1 and 2 are expressed in those interneurons from the MGE that migrate into the neocortex. MGE cells are repelled by the striatal semaphorins, thereby directing them away from the striatum.(51)
Ephrins and their receptors, the Eph tyrosine kinases, have also been implicated in neuronal migration. The B type ephrins are those with a transmembrane whereas the A type ephrins are membrane anchored through glycosylphosphatidylinositol lipid. EphB1-3 and EphA4 and their transmembrane ligands, ephrins-B2/3, are involved in SVZa migration.(52) When the extracellular parts of either EphB2 or ephrin-B2 were introduced into the lateral ventricle to inhibit the interaction between endogenous ephrin and Eph, the migration of SVZa cells was disrupted.(52)
The conclusion that axon guidance and neuronal migration share common mechanisms is supported not only by the findings of similar guidance cues, but also by further dissection of functional domains in the guidance cue Slit. The receptor for Slit is the transmembrane protein Roundabout (Robo).(39–41) It mediates Slit responses in both axon guidance(40,41,53) and neuronal migration.(37,43,54) Each mammalian Slit contains four leucine-rich repeats (LRRs), nine epidermal growth factor repeats, a laminin G domain and a cysteine-rich C-terminal region. Domain dissection studies have demonstrated that LRRs of Slit bind to Robo, and both the full-length Slit and the N-terminal regions of Slit, which has LRRs repel SVZa cells and olfactory bulb axons.(54,55) However, only the N terminus of Slit could induce branching of axons from the dorsal root ganglion cells, whereas the full-length Slit functions as a dominant negative manner in axon branching.(55) These similarities of Slit action in SVZa cell migration and axon repulsion further support the idea that axon guidance and neuronal migration share similar guidance mechanisms.(37,54)
Intracellular signal transduction pathways involved in neuronal migration
The signal transduction pathways for axon guidance have been recently reviewed, and the Rho family of small GTPases is clearly important (Fig. 2).(56,57) Interestingly, recent studies on intracellular signal transduction pathways suggest a possible fundamental conservation of molecular mechanisms guiding the neuronal migration and chemotactic leukocytes.(58–60) Multiple intracellular components in leukocyte chemotaxis have been identified as downstreams of the chemokine receptors (G-protein coupled seven transmembrane protein), and among these are the small GTPases of the Rho family.(58,59) Recently, a signal transduction pathway for Slit has been investigated for its role in neuronal migration,(60) and the Rho GTPase Cdc42 was found to be involved in neuronal migration. Taken together with results from studies of axon projection,(57,61) it is clear that the Rho GTPases are used in processes ranging from axon guidance to leukocyte chemotaxis (Fig. 2). We will review briefly here the signal transduction mechanism for Slit because it has been studied in the context in neuronal migration.
Figure 2.
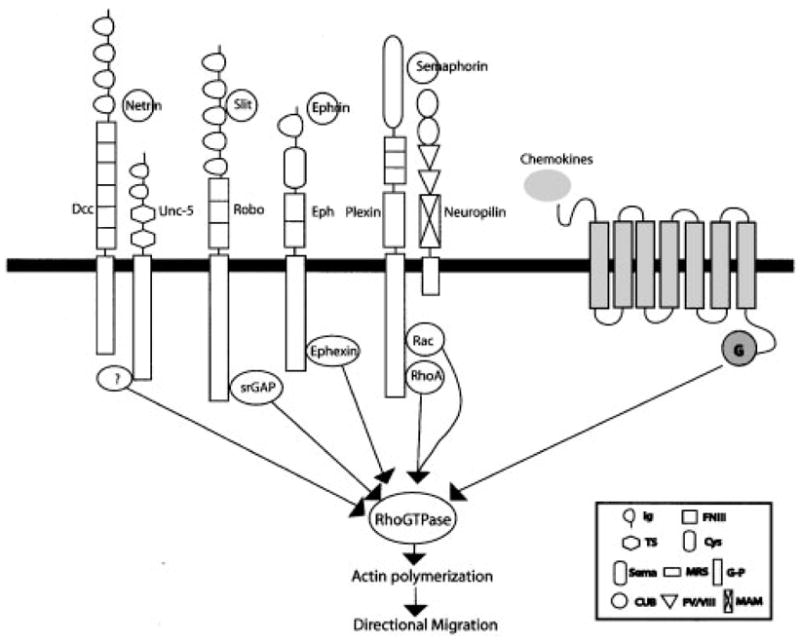
Rho GTPases signaling mechanisms of neuronal and leukocyte guidance cues. Several molecular linkages between receptors for neuronal guidance cues and Rho GTPases have been recently analyzed in vitro and in vivo. srGAP is a robo-binding GAP protein that principally inactivates cdc42.(60) Ephexin is a Eph-binding GEF that mediates ephrin-induced RhoA activation.(64) RhoA and Rac are known to directly bind to a semaphorin receptor, Plexin.(65, 66) The molecular linkage between netrin receptors and RhoGTP-ases has not been identified yet even though netrin regulates Rho GTPase activity in a heterologous system.(67) Abbreviations, DCC, Deleted in Colorectal Cancer; Robo, Roundabout; G, Heterotrimeric G proteins; Ig, Immunoglobulin domains; FNIII, Fibronectin type III repeats; TS, Thrombospondin type 1 repeat; Glb, globular; Cys, Cystein-rich; Sema, Semaphorin; MRS, Met-related sequence; G-P, Glycine-proline repeat; CUB, Complement binding; FV/VIII, Coagulation factor V/VIII homology; MAM, Meprin.
Robo contains five immunoglobulin (Ig) domains, three fibronectin type III domains, a single transmembrane domain and a cytoplasmic domain with four conserved motifs (CC0, CC1, CC2 and CC3).(53) It was previously shown that mutations in any one of the intracellular motifs can reduce, but do not eliminate, the function of Robo in axon guidance in Drosophila embryos.(53) CC1 is a tyrosine phosphorylation site for the Abelson (Abl) tyrosine kinase whereas CC1 and CC2 serve as a binding site for Enabled (Ena).(53) Abl and Ena interact with Robo genetically when analyzed in axon guidance phenotypes.(53) While the roles of Abl and Ena have not been determined in mammals or in neuronal migration, recent studies showing the role of mena, mammalian homologue of Ena, in cell migration(62) indicate that this molecular link may also play a role in vertebrate Slit-Robo pathways.
Recently, a signal transduction pathway for Slit has been specifically investigated for its role in neuronal migration,(60) and the identification of proteins binding to the CC3 motif has led to the proposal of a model that explains the repulsion caused by Slit.(60) The extracellular binding of Slit to Robo increases the binding of the intracellular CC3 motif of Robo to a new family of GTPase activation proteins named slit-robo GAPs (srGAP). The srGAPs inactivate the small GTPases of the Rho family, which includes Rho, Rac and Cdc42. In several cell types including the SVZa cells, Slit consistently inactivates Cdc42. When a constitutively active mutant form of Cdc42 was introduced into SVZa cells, these cells are no longer repelled by Slit.(60) This study clearly demonstrates a role for Cdc42 in mediating the repulsive response of SVZa to Slit. Because the Rho GTPases are known to promote actin polymerization,(63) the findings of their involvement in neuronal migration and axon guidance also suggest that actin polymerization is likely to be the common output of neuronal motility. The biochemical regulation of Rho and Rac by Slit seems to vary in different cell types and their functional significance in Slit response is unknown, although it is tempting to suggest that differential regulation of Cdc42, Rac and Rho may underlie different functions of Slit including axon repulsion and promotion of axon branching.
Conclusions
There is now compelling evidence for two differential modes of neuronal migration. Radial migration is responsible for the columnar organization in the cortex whereas tangential migration can add cellular complexity for more sophisticated cortical functions. The finding of guidance mechanisms shared among projecting axons, migrating neurons and chemotactic leukocytes suggests a fundamental mechanistic conservation among all somatic cells in the mammals, perhaps extending to free living metazoan cells. There are still many unanswered questions about neuronal migration. For example, for spatial control of neuronal migration, the functional significance of most of the endogenous guidance cues in neuronal migration has not been established although the effects of exogenous guidance cues are clear and the expression of the endogenous cues is highly suggestive. For temporal control of neuronal migration, mechanisms controlling either the timing or the speed of neuronal migration remain to be studied. At the cellular level, now that some neurons are known to migrate without glial fibers, the precise role of radial glia cells remains to be better understood. Intracellularly, we are only beginning to understand the signal transduction pathways involved in neuronal migration.
References
- 1.Bentivoglio M, Mazzarello P. The history of radial glia. Brain Res Bull. 1999;49:305–315. doi: 10.1016/s0361-9230(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 2.Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- 3.Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- 4.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 5.Rakic P, Sidman RL. Telencephalic origin of pulvinar neurons in the fetal human brain. Z Anat Entwicklungsgesch. 1969;129:53–82. doi: 10.1007/BF00521955. [DOI] [PubMed] [Google Scholar]
- 6.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 7.Corbin JG, Ne‘ry S, Fishell G. Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci. 2001;4(Supp 1):1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- 8.Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 9.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Yee KT, Simon HH, Tessier-Lavigne M, O’Leary DM. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24:607–622. doi: 10.1016/s0896-6273(00)81116-2. [DOI] [PubMed] [Google Scholar]
- 12.Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- 13.Caviness VS, Jr, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;148:141–151. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 14.Mariani J, Crepel F, Mikoshiba K, Changeux JP, Sotelo C. Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1977;281:1–28. doi: 10.1098/rstb.1977.0121. [DOI] [PubMed] [Google Scholar]
- 15.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 16.Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 18.D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 19.Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 20.Senzaki K, Ogawa M, Yagi T. Proteins of the CNR family are multiple receptors for Reelin. Cell. 1999;99:635–647. doi: 10.1016/s0092-8674(00)81552-4. [DOI] [PubMed] [Google Scholar]
- 21.Goldowitz D, Cushing RC, Laywell E, D’Arcangelo G, Sheldon M, Sweet HO, Davisson M, Steindler D, Curran T. Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of reelin. J Neurosci. 1997;17:8767–8777. doi: 10.1523/JNEUROSCI.17-22-08767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheldon M, Rice DS, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell BW, Cooper JA, Goldowitz D, Curran T. Scrambler and yotari disrupt the disabled gene and produce a reeler- like phenotype in mice. Nature. 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 23.Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 24.Howell BW, Herrick TM, Cooper JA. Reelin-induced tryosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- 26.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna XX, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhavan R, Tsai LH. A decade of cdk5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 28.Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- 30.Sobue K, Agarwal-Mawal A, Li W, Sun W, Miura Y, Paudel HK. Interaction of neuronal Cdc2-like protein kinase with microtubule- associated protein tau. J Biol Chem. 2000;275:16673–16680. doi: 10.1074/jbc.M000784200. [DOI] [PubMed] [Google Scholar]
- 31.Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase. Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- 32.Clark GD, Mizuguchi M, Antalffy B, Barnes J, Armstrong D. Predominant localization of the LIS family of gene products to Cajal- Retzius cells and ventricular neuroepithelium in the developing human cortex. J Neuropathol Exp Neurol. 1997;56:1044–1052. doi: 10.1097/00005072-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Bix GJ, Clark GD. Platelet-activating factor receptor stimulation disrupts neuronal migration In vitro. J Neurosci. 1998;18:307–318. doi: 10.1523/JNEUROSCI.18-01-00307.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faust PL, Hatten ME. Targeted deletion of the PEX2 peroxisome assembly gene in mice provides a model for Zellweger syndrome, a human neuronal migration disorder. J Cell Biol. 1997;139:1293–1305. doi: 10.1083/jcb.139.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 36.Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 37.Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothberg JM, Hartley DA, Walther Z, Artavanis-Tsakonas S. slit: an EGF-homologous locus of D.melanogaster involved in the development of the embryonic central nervous system. Cell. 1988;55:1047–1059. doi: 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- 39.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 40.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 41.Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 42.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y, Li H, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 44.Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 45.Kramer SG, Kidd T, Simpson JH, Goodman CS. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 2001;292:737–740. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- 46.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 47.Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 48.Murase S, Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci. 2002;22:3568–3579. doi: 10.1523/JNEUROSCI.22-09-03568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcantara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127:1359–1372. doi: 10.1242/dev.127.7.1359. [DOI] [PubMed] [Google Scholar]
- 50.Hamasaki T, Goto S, Nishikawa S, Ushio Y. A role of netrin-1 in the formation of the subcortical structure striatum: repulsive action on the migration of late-born striatal neurons. J Neurosci. 2001;21:4272–4280. doi: 10.1523/JNEUROSCI.21-12-04272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin- neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 52.Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 53.Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 54.Chen JH, Wen L, Dupuis S, Wu JY, Rao Y. The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci. 2001;21:1548–1556. doi: 10.1523/JNEUROSCI.21-05-01548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001;21:4281–4289. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 57.Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001;11:103–110. doi: 10.1016/s0959-4388(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 58.Allen WE, Zicha D, Ridley AJ, Jones GE. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol. 2002;14:196–202. doi: 10.1016/s0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, Wu JY, Xiong WC, Rao Y. Signal transduction in neuronal migration, roles of GTPase activating proteins and the small gtpase cdc42 in the slit-robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- 61.Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- 62.Bear JE, Loureiro JJ, Libova I, Fassler R, Wehland J, Gertler FB. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- 63.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 64.Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 65.Liu BP, Strittmatter SM. Semaphorin-mediated axonal guidance via Rho-related G proteins. Curr Opin Cell Biol. 2001;13:619–626. doi: 10.1016/s0955-0674(00)00260-x. [DOI] [PubMed] [Google Scholar]
- 66.Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11:339–344. doi: 10.1016/s0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 67.Li X, Saint-Cyr-Proulx E, Aktories K, Lamarche-Vane N. Rac1 and Cdc42 but Not RhoA or Rho Kinase Activities Are Required for Neurite Outgrowth Induced by the Netrin-1 Receptor DCC (Deleted in Colorectal Cancer) in N1E-115 Neuroblastoma Cells. J Biol Chem. 2002;277:15207–15214. doi: 10.1074/jbc.M109913200. [DOI] [PubMed] [Google Scholar]


