Abstract
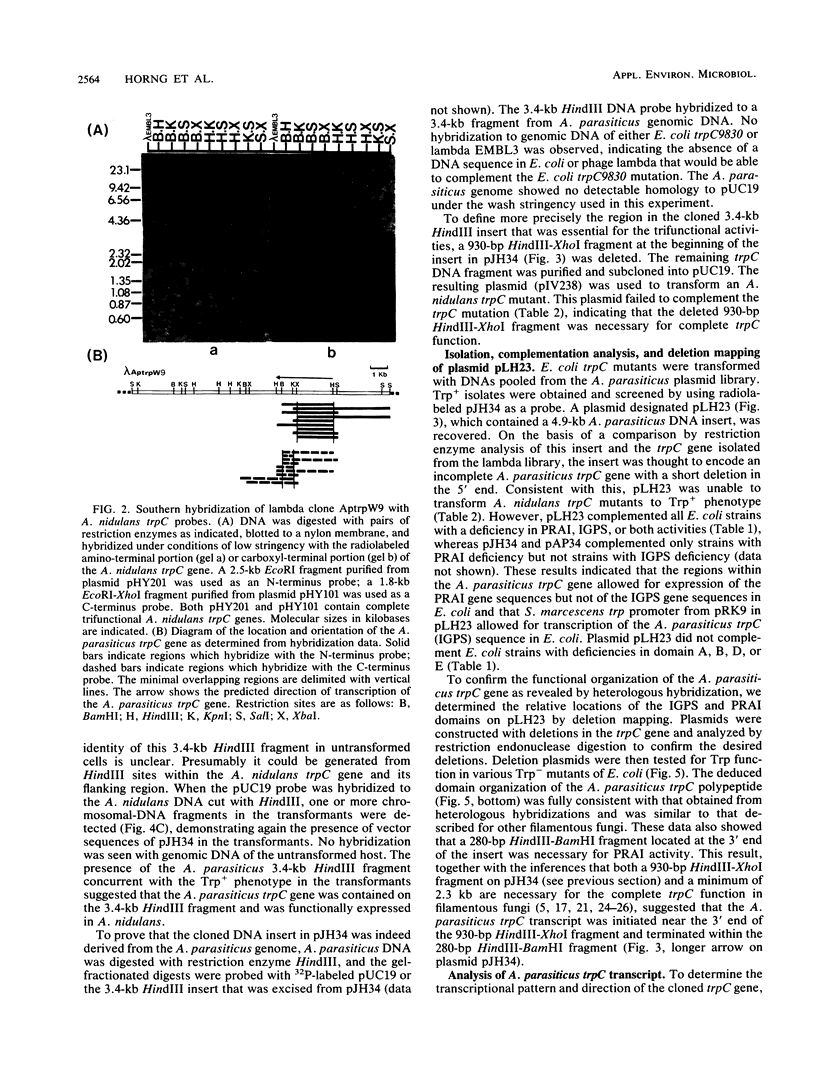
The trpC gene in the tryptophan biosynthetic pathway was isolated from an aflatoxigenic Aspergillus parasiticus by complementation of an Escherichia coli trpC mutant lacking phosphoribosylanthranilate isomerase (PRAI) activity. The cloned gene complemented an E. coli trpC mutant deficient in indoleglycerolphosphate synthase (IGPS) activity as well as an Aspergillus nidulans mutant strain that was defective in all three enzymatic activities of the trpC gene (glutamine amidotransferase, IGPS, and PRAI), thus indicating the presence of a complete and functional trpC gene. The location and organization of the A. parasiticus trpC gene on the cloned DNA fragment were determined by deletion mapping and by hybridization to heterologous DNA probes that were prepared from cloned trpC genes of A. nidulans and Aspergillus niger. These experiments suggested that the A. parasiticus trpC gene encoded a trifunctional polypeptide with a functional domain structure organized identically to those of analogous genes from other filamentous fungi. The A. parasiticus trpC gene was expressed constitutively regardless of the nutritional status of the culture medium. This gene should be useful as a selectable marker in developing a DNA-mediated transformation system to analyze the aflatoxin biosynthetic pathway of A. parasiticus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Choi H. T., Revuelta J. L., Sadhu C., Jayaram M. Structural organization of the TRP1 gene of Phycomyces blakesleeanus: implications for evolutionary gene fusion in fungi. Gene. 1988 Nov 15;71(1):85–95. doi: 10.1016/0378-1119(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Cihlar R. L., Sypherd P. S. The organization of the ribosomal RNA genes in the fungus Mucor racemosus. Nucleic Acids Res. 1980 Feb 25;8(4):793–804. [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hütter R., Niederberger P., DeMoss J. A. Tryptophan biosynthetic genes in eukaryotic microorganisms. Annu Rev Microbiol. 1986;40:55–77. doi: 10.1146/annurev.mi.40.100186.000415. [DOI] [PubMed] [Google Scholar]
- Kos T., Kuijvenhoven A., Hessing H. G., Pouwels P. H., van den Hondel C. A. Nucleotide sequence of the Aspergillus niger trpC gene: structural relationship with analogous genes of other organisms. Curr Genet. 1988 Feb;13(2):137–144. doi: 10.1007/BF00365648. [DOI] [PubMed] [Google Scholar]
- Käfer E. The anthranilate synthetase enzyme complex and the trifunctional trpC gene of Aspergillus. Can J Genet Cytol. 1977 Dec;19(4):723–738. doi: 10.1139/g77-079. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney E. J., Hamer J. E., Roberti K. A., Yelton M. M., Timberlake W. E. Primary structure of the trpC gene from Aspergillus nidulans. Mol Gen Genet. 1985;199(1):37–45. doi: 10.1007/BF00327506. [DOI] [PubMed] [Google Scholar]
- Muramatsu M. Preparation of RNA from animal cells. Methods Cell Biol. 1973;7:23–51. doi: 10.1016/s0091-679x(08)61770-7. [DOI] [PubMed] [Google Scholar]
- Muñoz-Rivas A. M., Specht C. A., Ullrich R. C., Novotny C. P. Isolation of the DNA sequence coding indole-3-glycerol phosphate synthetase and phosphoribosylanthranilate isomerase of Schizophyllum commune. Curr Genet. 1986;10(12):909–913. doi: 10.1007/BF00398288. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Rinehart J. E., Mitchell B. L., Oakley C. E., Carmona C., Gray G. L., May G. S. Cloning, mapping and molecular analysis of the pyrG (orotidine-5'-phosphate decarboxylase) gene of Aspergillus nidulans. Gene. 1987;61(3):385–399. doi: 10.1016/0378-1119(87)90201-0. [DOI] [PubMed] [Google Scholar]
- Revuelta J. L., Jayaram M. Phycomyces blakesleeanus TRP1 gene: organization and functional complementation in Escherichia coli and Saccharomyces cerevisiae. Mol Cell Biol. 1987 Aug;7(8):2664–2670. doi: 10.1128/mcb.7.8.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechtman M. G., Yanofsky C. Structure of the trifunctional trp-1 gene from Neurospora crassa and its aberrant expression in Escherichia coli. J Mol Appl Genet. 1983;2(1):83–99. [PubMed] [Google Scholar]
- Sánchez F., Touriño A., Traseira S., Pérez-Aranda A., Rubio V., Peñalva M. A. Molecular cloning and characterization of the trpC gene from Penicillium chrysogenum. Mol Gen Genet. 1986 Nov;205(2):248–252. doi: 10.1007/BF00430435. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Sequence of a yeast DNA fragment containing a chromosomal replicator and the TRP1 gene. Gene. 1980 Jul;10(2):157–166. doi: 10.1016/0378-1119(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Turgeon B. G., MacRae W. D., Garber R. C., Fink G. R., Yoder O. C. A cloned tryptophan-synthesis gene from the ascomycete Cochliobolus heterostrophus functions in Escherichia coli, yeast and Aspergillus nidulans. Gene. 1986;42(1):79–88. doi: 10.1016/0378-1119(86)90152-6. [DOI] [PubMed] [Google Scholar]
- Vogeli G., Horn E., Laurent M., Nath P. Recombinant DNA techniques: storage and screening of cDNA libraries with large numbers of individual colonies from initial transformations. Anal Biochem. 1985 Dec;151(2):442–444. doi: 10.1016/0003-2697(85)90202-7. [DOI] [PubMed] [Google Scholar]
- Woloshuk C. P., Seip E. R., Payne G. A., Adkins C. R. Genetic transformation system for the aflatoxin-producing fungus Aspergillus flavus. Appl Environ Microbiol. 1989 Jan;55(1):86–90. doi: 10.1128/aem.55.1.86-90.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., de Souza E. R., Mullaney E. J., Timberlake W. E. Developmental regulation of the Aspergillus nidulans trpC gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7576–7580. doi: 10.1073/pnas.80.24.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]