Abstract
β-amyloid (Aβ) is the primary protein component of senile plaques in Alzheimer’s disease and is believed to be associated with neurotoxicity in the disease. We and others have shown that Aβ binds with relatively high affinity to clustered sialic acid residues on cell surfaces and that removal of cell surface sialic acids attenuate Aβ toxicity. We have also shown that sialic acid functionalized dendrimeric polymers can act as mimics of cell surface sialic acid clusters and attenuate Aβ induced neurotoxicity. In the current study, we prepared sialic acid conjugated dendrimers using a physiologically relevant attachment of the sialic acid to the dendrimeric termini, and evaluated the Aβ toxicity attenuation properties of the dendrimers. We compared performance of sialic acid conjugated dendrimeric polymers in which the sialic acid moeties were attached to dendrimeric termini via the anomeric hydroxyl group of the sialic acid, a physiological attachment, to polymers in which the attachment was made via the carboxylic acid group on the sialic acid, a non-physiological attachment. This work enhances our understanding of Aβ-cell surface binding and is a step towards the development of new classes of sequestering agents as therapeutics for the prevention of Aβ toxicity in AD.
Keywords: Alzheimer’s disease, amyloid, dendrimer, sialic acid, toxicity
1. Introduction
Alzheimer’s disease (AD) is the leading cause of neurodegeneration in the United States, affecting approximately 4.5 million Americans in 2003 (Hebert et al., 2003), with an annual cost of care for these individuals estimated at over $100 billion (Ernst and Hay, 1994). The cost of care for patients also increases with progression of the disease (Zhu et al., 2006). One of the pathological hallmarks of AD is the formation of amyloid plaques in the cerebral cortex, the primary protein component of which is the 39–43 amino acid peptide β-amyloid (Aβ) (Selkoe, 1994). Aβ in a number of aggregated states including fibrils, protofibrils, and spherical oligomers, has been shown to be toxic to neurons and neuron-like cells in culture (Hartley et al., 1999; Seilheimer et al., 1997; Ward et al., 2000). It is believed that Aβ may play a major role in neurodegeneration associated with AD. To that end, agents which either sequester Aβ or interfere with Aβ interaction/binding to cells have been sought after as a means to reduce the pathological effects of Aβ (Drouet et al., 1999; Gelinas et al., 2004; Hertel et al., 1997; Hock et al., 2003; Matsuoka et al., 2003; Schenk et al., 2004).
A variety of evidence suggests that Aβ binds to cells via an interaction with surface glycolipids or glycoproteins (Ariga and Yu, 1999; Ariga et al., 2001; Avdulov et al., 1997; Choo-Smith et al., 1997; Kakio et al., 2001; Matsuzaki and Horikiri, 1999; McLaurin and Chakrabartty, 1996; McLaurin et al., 1998; Wakabayashi et al., 2005; Wang et al., 2001; Yanagisawa et al., 1995). Moreover, the sialic acid moiety is necessary for such interactions (Williamson et al., 2006). The affinity of the Aβ interaction increases when the gangliosides or sialic acid molecules on the cell surface are clustered (Kakio et al., 2001; Kakio et al., 2004). Based on these data, we had previously prepared sialic acid conjugated dendrimeric polymers of various sizes that mimicked the clustered sialic acid structure of the cell surface, and that bound with specificity to Aβ (Patel et al., 2006). We have shown that by introducing sialic acid-conjugated dendrimers to an in vitro system of SH-SY5Y neuroblastoma and Aβ, Aβ-induced neurotoxicity was attenuated. In these constructs, sialic acid molecules were attached using EDC chemistry to the amine termini of the dendrimers via the carboxylic acid group of the sialic acid. This attachment is different than that found on cell surface gangliosides in which sialic acid moieties are attached to the rest of the gangliosides via the anomeric hydroxyl group. In the current work, we constructed sialic acid functionalized dendrimers of similar sizes as before, but with the physiologically relevant attachment. Furthermore, we tested the ability of these constructs to attenuate Aβ-induced neurotoxicity and compared their efficacy to do so with sialic acid-dendrimer complexes made using the non-physiological attachment chemistry. Fig. 1 illustrates the differences in structure of these two constructs. Sialic acid dendrimer complexes made using the physiological attachment attenuated Aβ toxicity at lower concentrations than those made with the non-physiological attachment, although the binding affinity of the constructs for Aβ did not improve with physiological attachment of the sialic acid. These results could have implications for the design of new agents that bind pathogenic Aβ peptides for the treatment of neurodegenerative disease.
Figure 1.
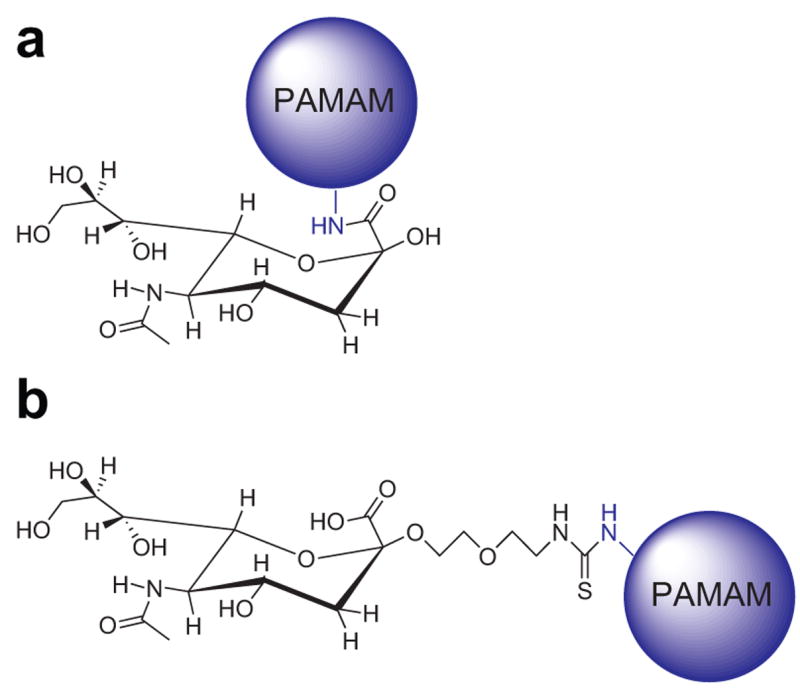
Two different attachments of the sialic acid moiety on the dendrimers: (a) non-physiological attachment of sialic acid to dendrimeric termini via carboxylic acid and (b) physiological attachment of sialic acid to dendrimeric termini via anomeric hydroxyl group.
2. Results
We have previously shown that artificial clusters of sialic acid that mimic cell surface sialic acid can act as decoy molecules for Aβ binding and attenuate Aβ induced neurotoxicity in vitro (Patel et al., 2006). The sialic acid moieties on those decoy molecules were attached to PAMAM dendrimers via the carboxylic acid group of the sialic acid (Fig. 1). Here, we prepared sialic acid conjugated dendrimers for use as Aβ binding decoy molecules in which the sialic acid moieties were attached to the dendrimers in a biologically relevant manner via the anomeric hydroxyl group of the sialic acid. We hypothesized that the biologically relevant attachment of sialic acid to dendrimers would improve the performance of the dendrimers in Aβ toxicity attenuation assays because the physiological attachment would lead to increased binding affinity of Aβ to dendrimers and decreased intrinsic toxicity of the sialic acid modified dendrimers.
Dendrimer Characterization
We used FTIR and NMR to confirm synthesis of the sialic acid conjugated dendrimers, shown in Figures 2 and 3, respectively. We used relative abundance of certain protons in the NMR spectra along with a colorimetric periodate-recorcinol assay to determine the percent of total end groups on the dendrimer that were sialic acid modified (Table 1). Using the physiological sialic acid attachment, a greater percent sialation was observed with the generation 4.0 dendrimer than the generation 3.0 dendrimer. Sialic acid conjugation was more efficient with the physiological attachment chemistry compared to the EDC chemistry used for the non-physiological attachment for the generation 4.0 dendrimer but not the generation 3.0 dendrimer.
Figure 2.
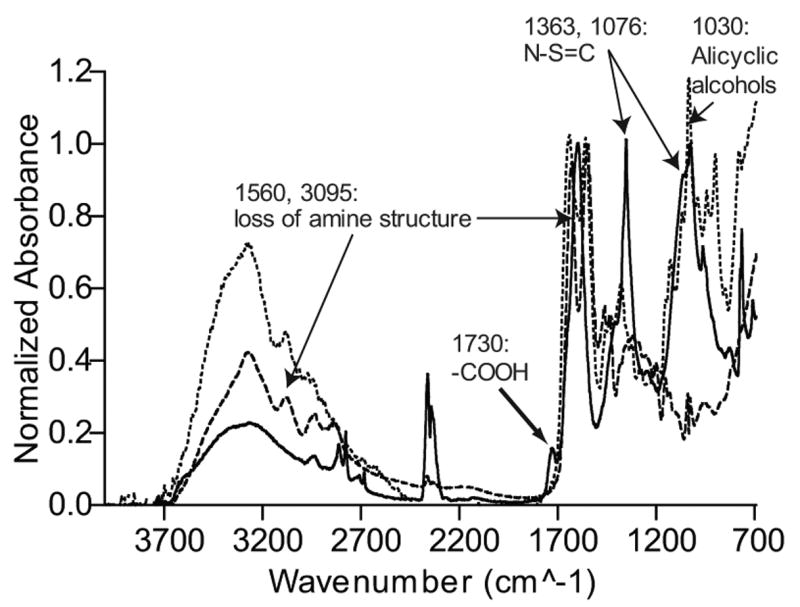
FTIR of conjugated and unconjugated PAMAM dendrimer generation 3.0. The dashed line represents the unconjugated dendrimer. The dotted line represents the sialic acid conjugated dendrimer attached to dendrimeric termini via the carboxylic acid group. The solid line represents sialic acid conjugated dendrimer attached via the anomeric hydroxyl group of the sialic acid. The –COOH peak can be seen at 1730 cm−1 indicating linkage of sialic acid via the hydroxyl group and not the carboxylic acid group.
Figure 3.
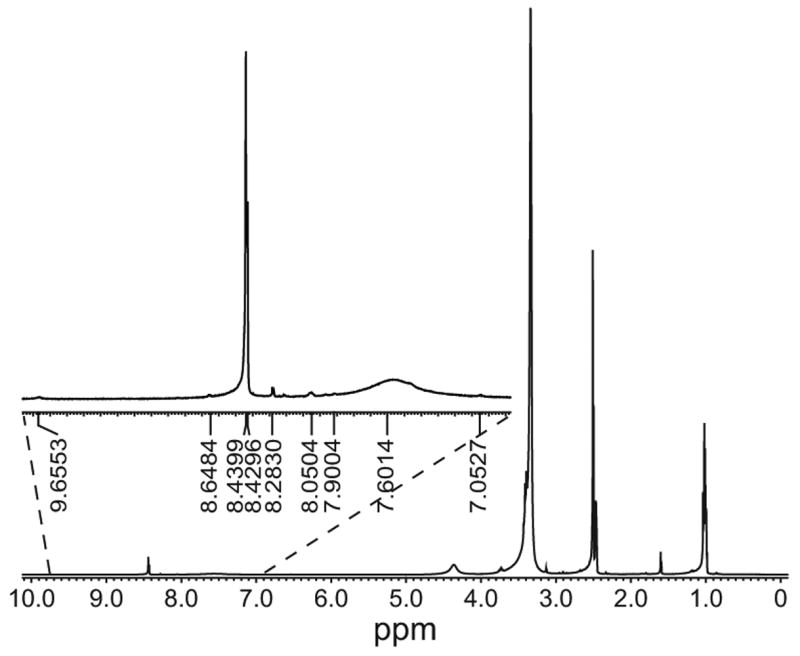
Proton NMR spectra of dendrimer sialic acid complex 4.0. Peaks ranging from 8.6 to 7.9 ppm represent the protons on the internal amides of the dendrimer. Peaks at 7.0., 7.6 and 9.6 represent the two protons in the linker (linked to the dendrimer termini but not to sialic acid), two linker protons (linked to dendrimer termini and sialic acid) and two linker protons (linker to dendrimeric termini and possibly a lactone derivative of the sialic acid).
Table 1.
Percentage conjugation of dendrimeric termini with sialic acid determined by proton NMR
| Percentage sialation
|
||
|---|---|---|
| Generation | -COOH attachment | -OH attachment* |
| 3.0 | 41.4 | 22.5 |
| 4.0 | 21.8 | 61.2 |
Percentages dendrimeric termini that were conjugated to the linker (not linked to sialic acid) and to linker (linked to a lactone derivative of sialic acid) were calculated to be 1.4 and 1.2 for dendrimer sialic acid complex 3.0, and 1.32 and 1.35 for generation 4.0 respectively.
Intrinsic toxicity of dendrimers
We examined the intrinsic toxicity of sialic acid dendrimers prepared using the physiological sialic acid attachment (Fig. 4). It is evident that for the same dendrimer concentrations, the generation 4.0 dendrimer-sialic acid complex is more toxic to the cells than the generation 3.0 conjugate. The LD50 values estimated from this data, along with the LD50 values of unconjugated dendrimers of the same size and those conjugated to sialic acid via the carboxylic acid group are compared in Table 2. Like the dendrimer sialic acid complexes conjugated via the carboxylic acid group, the newer constructs conjugated via the hydroxyl group had higher LD50 values, or lower intrinsic toxicity compared to the same-sized unconjugated dendrimers. Also, for all the dendrimers, unconjugated and conjugated, the LD50 values decreased with increase in generation number. For the generation 4.0 conjugate, physiological attachment of the sialic acid at the anomeric –OH resulted in a dendrimer conjugate that was less toxic than the conjugate prepared with the non-physiological attachment (at the –COOH). While sialic acid conjugation reduced the intrinsic toxicity of the generation 3.0 conjugates compared to the unmodified dendrimer, sialic acid attachment at the anomeric –OH did not significantly alter intrinsic toxicity of the generation 3.0 conjugate relative to the non-physiological attachment.
Figure 4.
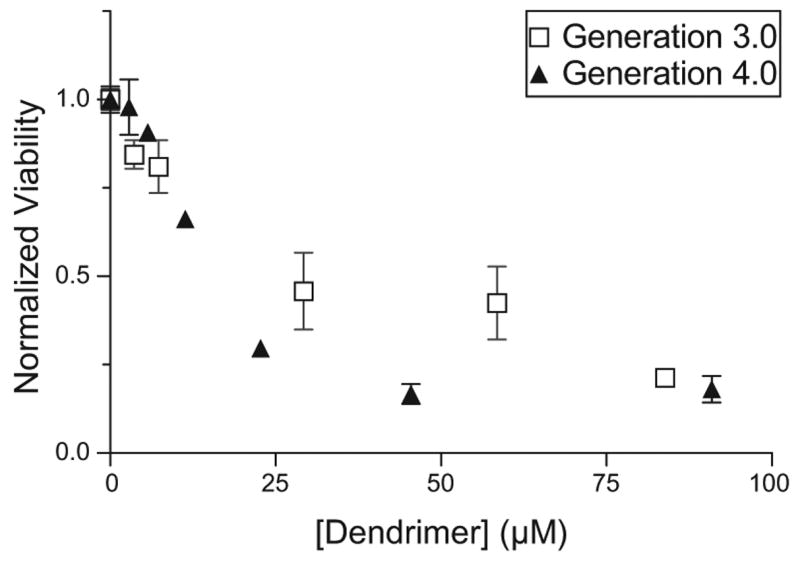
Intrinsic toxicity of sialic acid conjugated dendrimers 3.0 (open squares) and 4.0 (closed triangles) conjugated via the anomeric hydroxyl group.
Table 2.
LD50 values for unconjugated and sialic acid conjugated dendrimers. LD50 of sialic acid conjugated dendrimer conjugated at –COOH reprinted from Patel et al., 2006, with permission from Elsevier.
| LD50 (μM dendrimer)
|
|||
|---|---|---|---|
| Generation | Unconjugated | Conjugated via -COOH group | Conjugated via anomeric –OH group |
| 3.0 | 10 ± 7 | 42 ± 14 | 26.8 ± 9.6 |
| 4.0 | 1.7 ± 0.2 | 3.5 ± 1.2 | 16.4 ± 1.6 |
Sialic acid dendrimer – Aβ binding
To test whether physiological attachment of sialic acid to dendrimers would lead to enhanced Aβ binding affinity to sialic acid dendrimer conjugates, we prepared equilibrium binding isotherms of radioiodinated Aβ to immobilized dendrimers. Representative binding isotherms for generation 4.0 are shown in Fig. 5. In Table 3, equilibrium dissociation constants for Aβ to sialic acid modified dendrimers are shown. There were no statistically significant differences in Aβ equilibrium dissociation constants for the generation 3.0 dendrimer using the different sialic acid conjugation chemistries, while physiological attachment of sialic acid led to a modest increase in dissociation constant compared to the non-physiological attachment (or a reduction in binding affinity) for the generation 4.0 dendrimer. This result indicates that by conjugating sialic acid moieties to dendrimeric termini for use as Aβ binding decoy molecules via the anomeric hydroxyl group instead of the carboxylic acid group does not improve its Aβ binding affinity. Nonetheless, the binding affinities of all the dendrimer-sialic acid conjugates were on order of 10−8 M.
Figure 5.
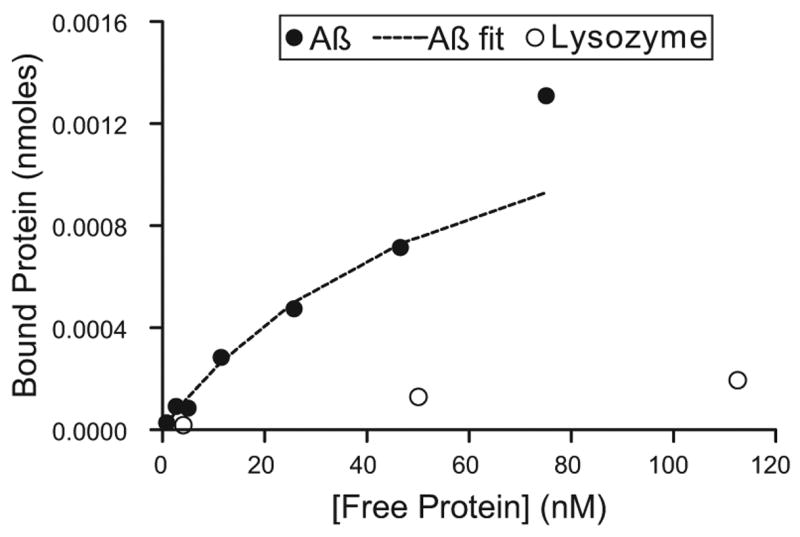
Representative equilibrium binding isotherms of Aβ (filled circles) and lysozyme (open circles) to dendrimer-sialic acid conjugate 4.0 with –OH linkage. The dashed line represents the fit of Eq. (2) to the data for Aβ binding.
Table 3.
Equilibrium dissociation constants for Aβ to different generations with sialic acid modification. Equilibrium constant for dendrimer conjugated via –COOH reprinted from Patel et al., 2006, with permission from Elsevier.
| KAβ (μM)
|
||
|---|---|---|
| Generation | -COOH attachment | -OH attachment |
| 3.0 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| 4.0 | 0.009 ± 0.005 | 0.05 ± 0.03 |
In addition to examining the binding of Aβ to sialic acid modified dendrimers, we also examined the binding of lysozyme, which is also known to form amyloid fibrils under some experimental conditions. It was found that like the sialic acid dendrimeric polymers prepared using the non-physiological attachment of sialic acid (Patel et al., 2006), the molecules prepared using a physiological attachment of sialic acid only bound lysozyme non-specifically (Fig. 5).
Attenuation of toxicity of Aβ by dendrimer-SA complexes
We analyzed the ability of the sialic acid functionalized dendrimers with the physiologically relevant sialic acid attachment to attenuate toxicity of 50 μM aggregated Aβ when added to differentiated SH-SY5Y neuroblastoma cells (Fig. 6). Typical cell viability after 24 hour treatment with 50 μM Aβ as prepared in our laboratory was between 60 and 75 %. Fig. 6 also shows the data for unconjugated dendrimer and the older dendrimer-sialic acid complexes prepared via the non-physiological attachment chemistry for comparison. It can be seen that for the generation 3.0 conjugate with the –OH linkage, the protective effect of the dendrimer-sialic acid complex increased with concentration up to around 1.0 μM sialic acid-dendrimer complex, and then started to decrease. Compared to the generation 3.0 conjugate with the –COOH sialic acid linkage, the conjugate with the –OH linkage maximally protected cells from Aβ toxicity at a lower concentration. But, the concentration of the –OH conjugate at which viability of cells decreased was also lower than concentration of the –COOH conjugate. This is corroborated by the lower LD50 value of the physiological (-OH) conjugate, compared to the non-physiological (-COOH) conjugate (Table 2). The same phenomenon was observed for the generation 4.0 molecules.
Figure 6.
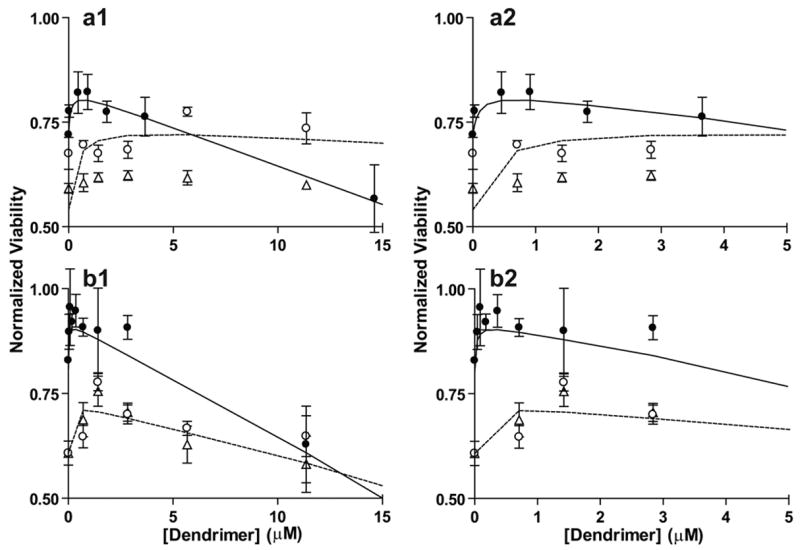
Normalized viability upon treatment with unconjugated dendrimer (open triangles), sialic acid conjugated dendrimers attached via carboxylic acid group (open circles), and sialic acid conjugated dendrimers attached via hydroxyl group (closed circles). (A) Generation 3.0 dendrimers. (B) Generation 4.0 dendrimers. The dashed line represents the model fit of Eq. (1) to sialic acid conjugated dendrimers attached via carboxylic acid group and the solid line represents the model fit to the data for the sialic acid conjugated dendrimer attached via the hydroxyl group. Viability data upon treatment with sialic acid conjugated dendrimers attached via carboxylic acid group adapted from Patel et al., 2006, with permission from Elsevier.
To quantify this behavior of increase in viability due the protective effect of sialic acid dendrimeric polymers from Aβ, the data in Fig. 6 was fit to Eq. 1 and the constants obtained for the newer conjugates with the –OH linkage were compared to those obtained for the older conjugated with the –COOH linkage. Table 4 displays the Aβ toxicity attenuation or inhibition constants, Ki, obtained for each dendrimer. It can be seen that for the new dendrimer-sialic acid complexes generation 3.0 and 4.0 linked via the anomeric hydroxyl group, the Aβ toxicity attenuation constant Ki decreased, compared to the older dendrimer sialic acid complexes conjugated via the carboxylic acid group of the sialic acid. These results suggest that while the sialic acid conjugated dendrimers prepared using a physiological sialic acid attachment were only slightly less toxic than dendrimers prepared with a non-physiological attachment of sialic acid, and had no significant improvement in binding affinity compared to the dendrimer prepared with a non-physiological attachment of sialic acid, these dendrimers attenuated the toxic effects of Aβ at significantly lower (a factor of 3 or more) concentrations of dendrimer.
Table 4.
Model constants for different generations of sialic acid-conjugated dendrimers. Constants for dendrimer conjugated via –COOH reprinted from Patel et al., 2006 with permission from Elsevier.
| Ki (μM)
|
||
|---|---|---|
| Generation | -COOH attachment | -OH attachment |
| 3.0 | 0.3 ± 0.005 | 0.1 ± 0.081 |
| 4.0 | 0.07 ± 0.002 | 0.024 ± 0.019 |
3. Discussion
We originally hypothesized that we could generate cell surface mimicking compounds by conjugating sialic acid to PAMAM dendrimers, and that the efficacy of these compounds in attenuating Aβ toxicity would be related to sialic acid valency, physiological attachment of the sialic acid, and intrinsic toxicity of the sialic acid dendrimer conjugate. In this work we compare data obtained from dendrimers in which sialic acids were conjugated at the anomeric –OH (physiological attachment) and at the carboxylic acid (non-physiological attachment).
We had hoped that the chemistry used in this work that produced a physiological sialic acid attachment to the dendrimers would result in increased sialation of dendrimers compared to EDC chemistry used in previous work (Patel et al., 2006), and thus reduce the instrinsic toxicity of the polymers. Others have shown that toxicity of these dendrimeric compounds is a function of both size or molecular weight of the molecule, and the charge on their termini (Choksakulnimitr et al., 1995; Fischer et al., 2003; Reuter et al., 1999; Wittmar et al., 2005). As seen in Table 1, only for the generation 4.0 dendrimer did we increase sialic acid conjugation relative to the dendrimers prepared with the non-physiological attachment of sialic acid. As seen in Table 2, the intrinsic toxicity of the newer sialic acid conjugated dendrimer generation 4.0 that was conjugated via the anomeric –OH group was slightly less cytotoxic than its counterpart conjugated via the –COOH group. This is not surprising given that the surface charge of the generation 4.0 molecule had been greatly reduced by the higher degree of sialic acid modification. With the same reasoning, it is apparent for generation 3.0 that the lower degree of modification achieved with the physiological (anomeric -OH) conjugation compared to the non-physiological (-COOH) attachment resulted in lesser dendrimer charge reduction and thus a more toxic construct.
We have previously shown that increasing the number of sialic acid moieties on a dendrimer resulted in a higher Aβ binding affinity and greater Aβ toxicity attenuation ability (Patel et al., 2006). This concept of improving a desired property in a molecule by multivalency has been nicely reviewed by Huskens (Huskens, 2006) and demonstrated by various groups in different biological systems (Kumar et al., 2006; Lees et al., 1994; Polizzotti and Kiick, 2006; Prieto et al., 2006; Woller and Cloninger, 2002). But this enhancement in Aβ binding with greater sialic acid valency was at the cost of increasing toxicity due to the larger size of dendrimers used to cluster more sialic acid. We had hoped that with a sialic acid attachment that was more biologically relevant, we would increase sialic acid conjugation efficiency, and thus increase valency without increasing size of dendrimer and the dendrimer intrinsic toxicity. We were only partially successful in increasing the sialic acid conjugation of the dendrimers (generation 4.0 only, Table 1). The increased conjugation efficiency of the generation 4.0 dendrimers with the physiological sialic acid attachment, and associated increased sialic acid valency, was associated with an increased ability to attenuate Aβ (Fig. 6, Table 4).
We had believed that physiological attachment of sialic acid relative to the non-physiological attachment of sialic acid to dendrimers would lead to greater Aβ binding to the dendrimers as some investigators have suggested that sialic binding proteins in general (Attrill et al., 2006) and Aβ in particular (Williamson et al., 2006) bind to the carboxylic acid on sialic acid via a positively charged amino acid residue. The same electrostatic interactions have been noted for sialic acids that are associated with gangliosides and the peptides that bind to those sialic acids (Ariga and Yu, 1999; Valdes-Gonzalez et al., 2001; Williamson et al., 2006). This electrostatic interaction would not occur with sialic acid modified dendrimeric polymers prepared with the –COOH attachment, but could occur in polymers prepared with the –OH sialic acid attachment. As seen in Fig. 5 and Table 3, while Aβ bound to dendrimers specifically and with an affinity at least an order of magnitude higher than what others have measured for Aβ binding to gangliosides (Ariga et al., 2001; Choo-Smith et al., 1997), there was no improvement in measured binding affinities of Aβ towards dendrimers with physiological sialic acid attachment compared to the non-physiological attachment. The lower dissociation constant or improved affinity seen with the sialic acid modified dendrimers compared to gangliosides may be due to the clustering of sialic acids on the dendrimeric polymers, or the combined effects of the electrostatic interaction of Aβ with the dendrimer backbone and the interaction of Aβ with the surface sialic acids on the dendrimer.
While there was no significant improvement in the ability of the same polymers to bind Aβ, there was a small improvement in ability of sialic acid polymers to attenuate Aβ toxicity when the sialic acid was attached to the polymer using a physiologically relevant chemistry as compared to the less relevant chemistry. There are several possible explanations for this apparent anomaly. We have assumed that the sialic acid conjugated dendrimers attenuated Aβ toxicity because of their ability to compete with the cell surface for Aβ binding. However, we have not examined Aβ binding and Aβ toxicity at the same concentration ranges. Low concentrations of Aβ were needed to get sensitive estimates of binding affinity, while higher concentrations of Aβ were needed in order to get reproducible estimates of Aβ toxicity. Thus, different physical phenomena could be occurring at the different concentration regimes. In particular, the aggregation properties of Aβ may change dramatically at the different concentrations. An alternate mechanism of action of sialic acid conjugated dendrimers than the one we propose (i.e. competitive binding of Aβ) is that the positively charged dendrimer associates with the negatively charged cell surface, thus preventing transport of the Aβ to the cell surface. If this alternate mechanism were responsible for the observed Aβ toxicity attenuation, we would expect to see toxicity attenuation regardless of the degree of sialic acid modification of the dendrimer and chemistry with which the sialic acid was attached. Based on our results, we can not discriminate between the two potential mechanisms of action of the sialic acid conjugated polymers.
We expected improved binding of Aβ to sialic acid dendrimers with the use of the physiological attachment of sialic acid, however, this improved binding affinity was not observed. We may have maintained the negative charge on the sialic acid that others suggest in important for Aβ binding by attaching the sialic acid to a dendrimer at the anomeric –OH. However, it is possible that we obscured any attractive electrostatic interactions of Aβ to sialic acid by attaching the sialic acid to a polycationic dendrimer. Moreover, it is possible that crowding sialic acid moieties led to a spatial arrangement that suboptimal for Aβ binding (Ariga et al., 2001). It is also possible that if the deprotecting or demethylation step in the chemistry was not complete, the negative charges of the sialic acid were not recovered, leading to a lower affinity for Aβ. Thus, we believe that with the use of a less highly charged polymer backbone and longer spacer between the charged polymer and the sialic acid, we may have seen greater Aβ binding affinity and greater toxicity attenuation properties of sialic acid polymers that employed a physiological attachment chemistry than seen in this work.
It is not clear from our data if monomeric, oligomer or more aggregated (fibril or protofibril) forms of Aβ bind to the sialic acid modified dendrimers. Work by Matsuzaki and coworkers indicate that fresh, not aggregated Aβ binds to cell surface gangliosides (Wakabayashi et al., 2005). On the contrary, work by others suggest that aggregated species bind gangliosides and that the oligomerization or fibrillization is independent of Aβ binding to gangliosides (Inaba et al., 2005; Williamson et al., 2006). In toxicity experiments reported here, Aβ was prepared such that there was always a mixture of both small and large Aβ oligomers.
There has been some controversy over the years as to the size of the toxic Aβ oligomer (Dahlgren et al., 2002; Kayed et al., 2003; Wang et al., 2002; Ward et al., 2000), confounded somewhat by the probability that Aβ aggregates when associated with cell membranes during the course of a toxicity experiment (Inaba et al., 2005). We can not address what form of Aβ binds to GM1 rich regions of cells and/or eventually leads to toxicity, nor can we address what population of Aβ species bind to sialic acid modified dendrimers prepared in experiments reported here.
In recent years there has been great interest and progress made in the development of molecular level strategies to target the formation, deposition, and cellular interaction of Aβ associated with neurotoxicity seen in Alzheimer’s disease (Blumberg, 1988; Etcheberrigaray et al., 2004, Blanchard et al., 2004; Ghanta et al., 1996, Gelinas et al., 2004; Hock et al., 2003; Mandavilli, 2006; Mount and Downton, 2006; Schenk et al., 2004). There is increasing evidence that molecules that sequester Aβ show mitigation in Aβ induced neurotoxicity (Drouet et al., 1999). Moreover, there is evidence that agents which sequester Aβ in the plasma may actually be effective at reducing Aβ levels in the cortex and may have the potential of reducing cognitive decline associated with disease (Bard et al., 2000; Bergamaschini et al., 2004; DeMattos et al., 2001; Matsuoka et al., 2003). In the current and previous works, we have demonstrated that sialic acid functionalized dendrimers that mimic cell surface sialic acid clusters can bind Aβ and attenuate Aβ induced neurotoxicity in vitro (Patel et al., 2006). In the current work, we demonstrated that attachment of sialic acid to dendrimeric termini via the anomeric hydroxyl group as opposed to the carboxylic acid group modestly enhances their ability to mitigate Aβ induced neurotoxicity. These findings add to our understanding of the Aβ sialic acid interaction and encourage further investigation into the development of sialic acid modified materials for use in prevention of Aβ toxicity.
4. Experimental Procedures
Materials
Aβ(1–40) was purchased from Biosource International (Camarillo, CA). Human neuroblastoma SH-SY5Y cells were purchased from ATCC (Manassas, VA). Cell dissociation buffer and cell culture reagents were purchased from Gibco-Invitrogen (Grand Island, NY). Propidium iodide (PI) was purchased from Molecular Probes (Eugene, OR). Human recombinant nerve growth factor – β (NGF-β), sialic acid (N-Acetylneuraminic acid, NANA) and polyamidoamine (PAMAM) dendrimer polymers generation 3.0 and 4.0 were purchased from Sigma-Aldrich (St. Louis, MO)., Iodo-Beads, G-5 desalting columns, Bolton-Hunter reagent (Sulfo-SHPP) and AminoLink Coupling Gel were purchased from Pierce Biotechnology (Rockford, IL). 125I was purchased from Amersham Biosciences (Piscataway, NJ). Ultrafiltration membranes were purchased from Millipore (Billerica, MA). All other chemicals were purchased from Sigma-Aldrich.
Peptide preparation
Aβ(1–40) stock solutions were prepared by dissolving the lyophilized peptide in anhydrous dimethyl sulfoxide (DMSO) to make 10 mg/ml stock solutions. After incubating for 30–45 minutes to 1h at 25°C, stock solutions of Aβ were diluted directly to their final concentrations in sterile cell culture medium and rotated at 25°C for 24 h prior to addition to cells. This method of peptide preparation yielded Aβ fibril and other aggregated species containing samples that were consistently toxic to cells in culture at concentrations between 20 and 100 μM (Lee et al., 2005; Lee et al., 2006; Patel et al., 2006; Rymer and Good, 2001).
Cell culture
Human neuroblastoma SH-SY5Y cells were cultured in a humidified 5% CO2/air incubator at 37°C in Minimum Essential Media (MEM), supplemented with 10% (vol/vol) fetal bovine serum, 2.2mg/ml NaHCO3, 100 U/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml amphotericin B (fungizone). SH-SY5Y cells were NGF differentiated prior to use in toxicity experiments by addition of 20 ng/ml NGF to cells for 5–7 days in 96 well plates.
Synthesis and purification of sialic acid-conjugated dendrimeric polymers
The procedure was adapted from work done by Woller et al. (Woller and Cloninger, 2002; Woller et al., 2003).
Acetylation of sialic acid (compound 1)
The acetyl protection was performed by first dissolving 250 mg of NANA in 10 ml of pyridine. To this solution was added 537 mg (497 μL) of acetic anhydride and 37 mg of 4-(dimethylamino)pyridine (DMAP). The solution was reacted at room temperature for 3 hours. After 3 hours, a 1 ml solution of hydrazine acetate in dimethyl formamide (DMF) was added to the pyridine solution. The mixture was reacted at 55°C for 15 min. 1 ml trichloroacetonitrile and 45.3 μl of 1,8- diazabicyclo[5.4.0]undec-7-ene (DBU) were then added, and the system was reacted at 0°C for 1.5 hours. The final solution was stored at −80°C until used.
Production of 2-(2-isothiocyanatoethoxy ethanol) (compound 2)
A solution containing 700 μl of 2-(2-aminoethoxy)ethanol and 2 ml of triethylamine in 10 ml of chloroform was added slowly over the course of an hour to a stirred solution containing 540 μl of thiophosgene in 40 ml of chloroform. The solvent was removed in vacuo. The resulting residue was dissolved in 10 ml of deionized water. A two-step extraction was performed using two 10 ml volumes of dichloromethane. The organic phases from the extraction were combined and washed twice with deionized water (2 × 10 ml). The organic layer was then dried over magnesium sulfate and filtered. The solvent was removed in vacuo. The resulting yellowish oil was stored at −80°C until used.
Attachment of compound 2 to dendrimer (compound 3)
60 μl (2.89 μmoles) of the generation 3.0 dendrimer or 107 μl (0.75 μmoles) solution of generation 4.0 polyamidoamine (PAMAM) (0.57 μmol of dendrimer) was evaporated under reduced pressure. The resulting residue was dissolved in 5 ml of dimethyl sulfoxide (DMSO). A 1.5-fold molar excess of compound 2 was added to the DMSO mixture (this excess is based on the number of terminal amines available on the dendrimer and not the amount of dendrimer). The solution was reacted at room temperature for 8 hours. The solvent was removed in vacuo.
Attachment of sialic acid (compound 4)
The residue of compound 3 was dissolved in 20 ml of dichloromethane with 80 mg of 4Å molecular sieves under nitrogen. To the solution, equimolar quantities of (to dendrimeric terminii) of compound 1 and 200μL boron trifluoride-diethyl etherate was added slowly. The solution was reacted at room temperature for 7 hours. Sodium bicarbonate (100 mg) was added to the solution, and the solution was filtered over celite 545 filter agent. The solvent was removed in vacuo. The resulting compound was stored at −80°C until used.
Deprotection of sialic acid
Compound 4 was dissolved in 20 ml of a methanol/deionized water mixture (1:1). Methanolic base (1 M, 180 μl or till pH reached 9.5) was added, and the solution was mixed overnight until all solid was dissolved. The solution was neutralized with Amberlite IR-120 and filtered using a glass filter. The solvent was removed in vacuo. The resulting residue was dissolved in deionized water, and purified using 10 kDa centrifuge filter units. The resulting solution was stored at 4°C until used.
Verification and quantification of extent of sialic acid conjugation
Verification of the presence of sialic acid on dendrimers was done by FT-IR spectroscopy using a System 2000 FTIR (Perkin Elmar, Norwalk, CT). The FTIR spectra of the unconjugated and conjugated dendrimers with both attachments are shown in Fig. 2. It is apparent by the presence of certain characteristic structures (thiourea, carboxylic acids, and alicyclic alcohols) and the loss of other peaks (amine structure) that the chemistry was successful. Moreover, the spectra for the sialic acid conjugated dendrimers with the anomeric hydroxyl group attachment shows the carboxylic acid group peak at 1730 cm−1 that the older constructs do not show, suggesting that there are indeed, unmodified –COOH groups on the newer constructs.
Conjugation to sialic acid was also confirmed by the Periodate-Recorcinol assay that estimates the amount of sialic acid in the sample (Bhavanandan and Sheykhnazari, 1993). Samples were diluted in 0.1 N sulfuric acid and containing 0.2% sodium dodecyl sulphate. Hydrolysis of the sialic acid moieties was carried out at 80°C for 1h. After samples were allowed to equilibrate to room temperature, 40 μl of the samples were mixed with 10 μl periodic acid solution (0.032 M), shaken for 5 min and kept on ice for 35 min. Then, 100 μl of recorcinol solution (0.6 g in 100ml 6 N HCl containing 25 μmol copper sulphate) was added. The samples were then covered and kept at 80°C for 1h. After adding 100 μl of tert-butyl alcohol to the samples, their absorbances were read at 650 nm as an estimate of amount of sialic acid present. The percentage sialation found in the generation 3.0 and 4.0 dendrimer sialic acid complexes were found to be 51 ± 3 and 56 ± 9 respectively. The thiol group present on the linker may have interfered in the assay. Thus, for confident estimates of percentages of sialic acid labeling, proton NMR was utilized. The solvent in the dendrimer solutions were evaporated using the Savant Speed Vac Plus concentrator (Cambridge Scientific Products, Cambridge, MA). The material was then dissolved in d6-dimethyl sulphoxide to a concentration of roughly 5 mg/ml for 4 h prior to NMR scanning using the Joel ECX400 NMR system. 1024 scans were taken for each sample. Fig. 3 shows the NMR spectra of the generation 4.0 dendrimer sialic acid complex. The peaks at 7.09 ppm represent the protons neighboring the thiol group (NHCSNH) on linkers attached to dendrimeric termini but not to sialic acid. The peak at 7.6 ppm represents the protons neighboring the thiol group (NHCSNH) on linkers attached to both, dendrimeric termini and sialic acid. The peaks from 7.9–8.4 ppm represent the internal amides of the dendrimers. Table 1 shows the percentage sialation of the sialic acid functionalized dendrimers with the two different attachments for the different generations of dendrimers used. The low degree of sialic acid labeling for generation 3.0 may be attributed to the possible inhomogeneity of the dendrimer. The structural defects may have precluded a higher percentage labeling.
PI toxicity assay
Cell toxicity was measured as previously described (Patel et al., 2006). NGF-diiferenciated SH-SY5Y cells were stained with PI, a nucleic acid dye that stains cells with permeable membranes. In all experiments, Aβ was added to cells approximately 30 minutes prior to the addition of dendrimer-sialic acid conjugates. 24 hours after addition of Aβ or other agent, cells were prepared for viability measurement as before using the FACSArray Bioanalyzer (Becton-Dickonson, Bedford, MA). Gating was done so as to obtain percentages of the total cell population that were viable. Normalized viability values were obtained by dividing the percentage of viable cells in the sample by that in the control samples with no Aβ or other agent.
Estimation of LD50 values and toxicity inhibition parameters
LD50 values, defined here as concentrations of dendrimer-sialic acid conjugate that led to a 50% reduction in cell viability, were obtained by linear interpolation of viability as a function of concentration data about the region of 50% viability. The uncertainty of the concentration of dendrimer was estimated by determining the 95% confidence interval of concentration from the viability curves, and using that to determine a coefficient of variation of the measurement.
We estimated toxicity inhibition constants for sialic acid dendrimers by fitting Equation (1) to viability using a non-linear least squares regression method (KaleidaGraph 3.6, Synergy Software, Reading, PA) (Patel et al., 2006).
| (1) |
V is the normalized viability of the cell sample treated with 50 μM Aβ and dendrimer-sialic acid conjugate. Vo is the normalized viability of the cell sample treated with 50 μM Aβ and zero dendrimer-sialic acid conjugate. Vmax is the maximum normalized viability attainable if treatment with sialic acid modified dendrimer totally protected cells from Aβ. Vmax approaches a theoretical limit of 100%. Ki and kt are empirical constants associated with sialic acid binding to Aβ (and inhibition of its toxicity by sequestering Aβ), and with the intrinsic toxicity of the dendrimer conjugates, respectively.
Radiochemical Binding assay
Binding of Aβ to sialic acid conjugated dendrimers was determined as previously done using radiochemical techniques. Aβ(1–40) was radioiodinated via a modified Bolton Hunter method to preferentially label at the N terminus of the peptide. Labeling at other primary amines was inhibited by maintaining the pH below the pKa of those residues. 100 nmol of sulfo-SHPP was iodinated with 200 μCi of 125I using the IodoBead catalyst for 15 minutes at pH 8.0 borate buffer in a volume of 140 μL. After removal of the catalyst, 2 nmol (100 μL) of freshly prepared β-amyloid(1–40) (in water) was added and allowed to react to the iodinated sulfo-SHPP for 3 hours at 4°C. The resulting condensation product was separated from free 125I using a G-5 desalting column, employing phosphate buffer as the eluent. By precipitation of the peptide with 20 wt% trichloroacetic acid in the presence of 1 mg/ml bovine serum albumin, the precipitatable activity was determined to be 80%. Peptide concentration was determined from the activity of the 125I-labeled Aβ by assuming that the peptide was recovered from the desalting column using one column volume of the eluting buffer and that the peptide concentration was proportional to the precipitatable activity of the fraction. No attempt was made to remove unlabeled peptide from labeled peptide. Relative aggregation state of the peptide was assessed using native polyacrylamide gel electrophoresis, counting activity of sliced sections of gel to determine relative abundance of different molecular weight species. The iodinated peptide was approximately 60% monomer or dimer, with the balance being large (above 100 kDa) aggregate.
Lysozyme was iodinated directly without using sulfo-SHPP. Two Iodobeads were added to 100 μL PBS, to which 100 μCi 125I (10μl) were added. 390 μL of 1mg/ml of lysozyme was incubated in the presence of 125I and Iodobeads for 10 minutes. Iodinated protein was separated from free 125I using G-5 desalting columns. Iodinated protein concentration was determined from UV absorbance at 280 nm. The dendrimer-sialic acid complexes were immobilized to AminoLink agarose coupling gels that were activated to form aldehyde functional groups reactive with primary amines. These gels were used in radiochemical binding assays within 10 days of preparation.
Binding assays were carried out by incubating 125I-labeled protein of different concentrations (nanomolar level) with 10 μL of the immobilized dendrimer-sialic acid complexes (~10 μM end concentration) of different generations at room temperature for 2 h. Tween-20 was also added at a final concentration of 0.1 % to block nonspecific binding. Thereafter, free Aβ was removed from that bound to the immobilized gel by centrifugation at 12,000 g for 5 minutes. The activities of both bound and unbound Aβ were counted separately using a Wallac MicroBeta TriLux microplate liquid scintillation counter (Perkin Elmer, Wellesley, MA). Equilibrium binding isotherms for binding to immobilized dendrimers or sialic acid conjugated dendrimers were corrected for binding of protein to unmodified agarose gel. Binding constants were determined from these resultant equilibrium binding isotherms by fitting a langmuir isotherm (Equation 2) to the data using a non-linear least squares regression algorithm in KaleidaGraph.
| (2) |
where Cabs is the amount of bound Aβ to the dendrimer-gel, Cab is the concentration of free Aβ, Ct is the total Aβ binding sites on the surface of the gel, and KAβ is the equilibrium dissociation constant (M).
Supplementary Material
Supplemental Figure. Synthesis of sialic acid functionalized dendrimers sialated at anomeric –OH position of sialic acid
Acknowledgments
We would like thank Dr. Mary Cloninger for her guidance with the chemistry. The study was supported by grants from the NIH (NS050346, AG025586).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ariga T, Yu RK. GM1 inhibits amyloid beta-protein-induced cytokine release. Neurochem Res. 1999;24:219–26. doi: 10.1023/a:1022557920150. [DOI] [PubMed] [Google Scholar]
- Ariga T, Kobayashi K, Hasegawa A, Kiso M, Ishida H, Miyatake T. Characterization of high-affinity binding between gangliosides and amyloid beta-protein. Arch Biochem Biophys. 2001;388:225–30. doi: 10.1006/abbi.2001.2304. [DOI] [PubMed] [Google Scholar]
- Attrill H, Takazawa H, Witt S, Kelm S, Isecke R, Brossmer R, Ando T, Ishida H, Kiso M, Crocker PR, van Aalten DM. The structure of siglec-7 in complex with sialosides: leads for rational structure-based inhibitor design. Biochem J. 2006;397:271–8. doi: 10.1042/BJ20060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdulov NA, Chochina SV, Igbavboa U, Warden CS, Vassiliev AV, Wood WG. Lipid binding to amyloid beta-peptide aggregates: preferential binding of cholesterol as compared with phosphatidylcholine and fatty acids. J Neurochem. 1997;69:1746–52. doi: 10.1046/j.1471-4159.1997.69041746.x. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bergamaschini L, Rossi E, Storini C, Pizzimenti S, Distaso M, Perego C, De Luigi A, Vergani C, De Simoni MG. Peripheral treatment with enoxaparin, a low molecular weight heparin, reduces plaques and beta-amyloid accumulation in a mouse model of Alzheimer’s disease. J Neurosci. 2004;24:4181–6. doi: 10.1523/JNEUROSCI.0550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavanandan VP, Sheykhnazari M. Adaptation of the periodate-resorcinol method for determination of sialic acids to a microassay using microtiter plate reader. Anal Biochem. 1993;213:438–40. doi: 10.1006/abio.1993.1445. [DOI] [PubMed] [Google Scholar]
- Blanchard BJ, Chen A, Rozeboom LM, Stafford KA, Weigele P, Ingram VM. Efficient reversal of Alzheimer’s disease fibril formation and elimination of neurotoxicity by a small molecule. Proc Natl Acad Sci U S A. 2004;101:14326–32. doi: 10.1073/pnas.0405941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg PM. Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture. Cancer Res. 1988;48:1–8. [PubMed] [Google Scholar]
- Choksakulnimitr S, Masuda S, Tokuda H, Takakura Y, Hashida M. In vitro cytotoxicity of macromolecules in different cell culture systems. J Control Release. 1995;34:233–241. [Google Scholar]
- Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by specific binding of Abeta-(1–40) peptide to ganglioside-containing membrane vesicles. J Biol Chem. 1997;272:22987–90. doi: 10.1074/jbc.272.37.22987. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–53. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet B, Pincon-Raymond M, Chambaz J, Pillot T. Laminin 1 attenuates beta-amyloid peptide Abeta(1–40) neurotoxicity of cultured fetal rat cortical neurons. J Neurochem. 1999;73:742–9. doi: 10.1046/j.1471-4159.1999.0730742.x. [DOI] [PubMed] [Google Scholar]
- Ernst RL, Hay JW. The US economic and social costs of Alzheimer’s disease revisited. Am J Public Health. 1994;84:1261–4. doi: 10.2105/ajph.84.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van der Auwera I, Wera S, Qiao L, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci U S A. 2004;101:11141–6. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–31. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- Gelinas DS, DaSilva K, Fenili D, St George-Hyslop P, McLaurin J. Immunotherapy for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14657–62. doi: 10.1073/pnas.0404866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta J, Shen CL, Kiessling LL, Murphy RM. A strategy for designing inhibitors of beta-amyloid toxicity. J Biol Chem. 1996;271:29525–8. doi: 10.1074/jbc.271.47.29525. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–84. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hertel C, Terzi E, Hauser N, Jakob-Rotne R, Seelig J, Kemp JA. Inhibition of the electrostatic interaction between beta-amyloid peptide and membranes prevents beta-amyloid-induced toxicity. Proc Natl Acad Sci U S A. 1997;94:9412–6. doi: 10.1073/pnas.94.17.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–54. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Huskens J. Multivalent interactions at interfaces. Curr Opin Chem Biol. 2006;10:537–43. doi: 10.1016/j.cbpa.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Inaba S, Okada T, Konakahara T, Kodaka M. Specific binding of amyloid-beta-protein to IMR-32 neuroblastoma cell membrane. J Pept Res. 2005;65:485–90. doi: 10.1111/j.1399-3011.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid beta-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem. 2001;276:24985–90. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- Kakio A, Yano Y, Takai D, Kuroda Y, Matsumoto O, Kozutsumi Y, Matsuzaki K. Interaction between amyloid beta-protein aggregates and membranes. J Pept Sci. 2004;10:612–21. doi: 10.1002/psc.570. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kumar PV, Asthana A, Dutta T, Jain NK. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J Drug Target. 2006;14:546–56. doi: 10.1080/10611860600825159. [DOI] [PubMed] [Google Scholar]
- Lee S, Carson K, Rice-Ficht A, Good T. Hsp20, a novel alpha-crystallin, prevents Abeta fibril formation and toxicity. Protein Sci. 2005;14:593–601. doi: 10.1110/ps.041020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Carson K, Rice-Ficht A, Good T. Small heat shock proteins differentially affect Abeta aggregation and toxicity. Biochem Biophys Res Commun. 2006;347:527–33. doi: 10.1016/j.bbrc.2006.06.128. [DOI] [PubMed] [Google Scholar]
- Lees WJ, Spaltenstein A, Kingery-Wood JE, Whitesides GM. Polyacrylamides bearing pendant alpha-sialoside groups strongly inhibit agglutination of erythrocytes by influenza A virus: multivalency and steric stabilization of particulate biological systems. J Med Chem. 1994;37:3419–33. doi: 10.1021/jm00046a027. [DOI] [PubMed] [Google Scholar]
- Mandavilli A. The amyloid code. Nat Med. 2006;12:747–51. doi: 10.1038/nm0706-747. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Saito M, LaFrancois J, Saito M, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K, Horikiri C. Interactions of amyloid beta-peptide (1–40) with ganglioside-containing membranes. Biochemistry. 1999;38:4137–42. doi: 10.1021/bi982345o. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem. 1996;271:26482–9. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Franklin T, Fraser PE, Chakrabartty A. Structural transitions associated with the interaction of Alzheimer beta-amyloid peptides with gangliosides. J Biol Chem. 1998;273:4506–15. doi: 10.1074/jbc.273.8.4506. [DOI] [PubMed] [Google Scholar]
- Mount C, Downton C. Alzheimer disease: progress or profit? Nat Med. 2006;12:780–4. doi: 10.1038/nm0706-780. [DOI] [PubMed] [Google Scholar]
- Patel D, Henry J, Good T. Attenuation of beta-amyloid induced toxicity by sialic acid-conjugated dendrimeric polymers. Biochim Biophys Acta. 2006;1760:1802–9. doi: 10.1016/j.bbagen.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Polizzotti BD, Kiick KL. Effects of polymer structure on the inhibition of cholera toxin by linear polypeptide-based glycopolymers. Biomacromolecules. 2006;7:483–90. doi: 10.1021/bm050672n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto MJ, Bacigalupe D, Pardini O, Amalvy JI, Venturini C, Morilla MJ, Romero EL. Nanomolar cationic dendrimeric sulfadiazine as potential antitoxoplasmic agent. Int J Pharm. 2006;326:160–8. doi: 10.1016/j.ijpharm.2006.05.068. [DOI] [PubMed] [Google Scholar]
- Reuter JD, Myc A, Hayes MM, Gan Z, Roy R, Qin D, Yin R, Piehler LT, Esfand R, Tomalia DA, Baker JR., Jr Inhibition of viral adhesion and infection by sialic-acid-conjugated dendritic polymers. Bioconjug Chem. 1999;10:271–8. doi: 10.1021/bc980099n. [DOI] [PubMed] [Google Scholar]
- Rymer DL, Good TA. The role of G protein activation in the toxicity of amyloidogenic Abeta-(1–40), Abeta-(25–35), and bovine calcitonin. J Biol Chem. 2001;276:2523–30. doi: 10.1074/jbc.M005800200. [DOI] [PubMed] [Google Scholar]
- Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Curr Opin Immunol. 2004;16:599–606. doi: 10.1016/j.coi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Seilheimer B, Bohrmann B, Bondolfi L, Muller F, Stuber D, Dobeli H. The toxicity of the Alzheimer’s beta-amyloid peptide correlates with a distinct fiber morphology. J Struct Biol. 1997;119:59–71. doi: 10.1006/jsbi.1997.3859. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–47. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Siebert HC, Born K, Andre S, Frank M, Kaltner H, von der Lieth CW, Heck AJ, Jimenez-Barbero J, Kopitz J, Gabius HJ. Carbohydrate chain of ganglioside GM1 as a ligand: identification of the binding strategies of three 15 mer peptides and their divergence from the binding modes of growth-regulatory galectin-1 and cholera toxin. Chem Eur J. 2005;12:388–402. doi: 10.1002/chem.200500505. [DOI] [PubMed] [Google Scholar]
- Valdes-Gonzalez T, Inagawa J, Ido T. Neuropeptides interact with glycolipid receptors: a surface plasmon resonance study. Peptides. 2001;22:1099–106. doi: 10.1016/s0196-9781(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Wakabayashi M, Okada T, Kozutsumi Y, Matsuzaki K. GM1 ganglioside-mediated accumulation of amyloid beta-protein on cell membranes. Biochem Biophys Res Commun. 2005;328:1019–23. doi: 10.1016/j.bbrc.2005.01.060. [DOI] [PubMed] [Google Scholar]
- Wang SS, Rymer DL, Good TA. Reduction in cholesterol and sialic acid content protects cells from the toxic effects of beta-amyloid peptides. J Biol Chem. 2001;276:42027–34. doi: 10.1074/jbc.M102834200. [DOI] [PubMed] [Google Scholar]
- Wang SS, Becerra-Arteaga A, Good TA. Development of a novel diffusion-based method to estimate the size of the aggregated Abeta species responsible for neurotoxicity. Biotechnol Bioeng. 2002;80:50–9. doi: 10.1002/bit.10347. [DOI] [PubMed] [Google Scholar]
- Ward RV, Jennings KH, Jepras R, Neville W, Owen DE, Hawkins J, Christie G, Davis JB, George A, Karran EH, Howlett DR. Fractionation and characterization of oligomeric, protofibrillar and fibrillar forms of beta-amyloid peptide. Biochem J. 2000;348(Pt 1):137–44. [PMC free article] [PubMed] [Google Scholar]
- Williamson MP, Suzuki Y, Bourne NT, Asakura T. Binding of amyloid beta-peptide to ganglioside micelles is dependent on histidine-13. Biochem J. 2006;397:483–90. doi: 10.1042/BJ20060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmar M, Ellis JS, Morell F, Unger F, Schumacher JC, Roberts CJ, Tendler SJ, Davies MC, Kissel T. Biophysical and transfection studies of an amine-modified poly(vinyl alcohol) for gene delivery. Bioconjug Chem. 2005;16:1390–8. doi: 10.1021/bc0500995. [DOI] [PubMed] [Google Scholar]
- Woller EK, Cloninger MJ. The lectin-binding properties of six generations of mannose-functionalized dendrimers. Org Lett. 2002;4:7–10. doi: 10.1021/ol016568+. [DOI] [PubMed] [Google Scholar]
- Woller EK, Walter ED, Morgan JR, Singel DJ, Cloninger MJ. Altering the strength of lectin binding interactions and controlling the amount of lectin clustering using mannose/hydroxyl-functionalized dendrimers. J Am Chem Soc. 2003;125:8820–6. doi: 10.1021/ja0352496. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer’s disease. Nat Med. 1995;1:1062–6. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- Zhu CW, Scarmeas N, Torgan R, Albert M, Brandt J, Blacker D, Sano M, Stern Y. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology. 2006;67:998–1005. doi: 10.1212/01.wnl.0000230160.13272.1b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Synthesis of sialic acid functionalized dendrimers sialated at anomeric –OH position of sialic acid


