Abstract
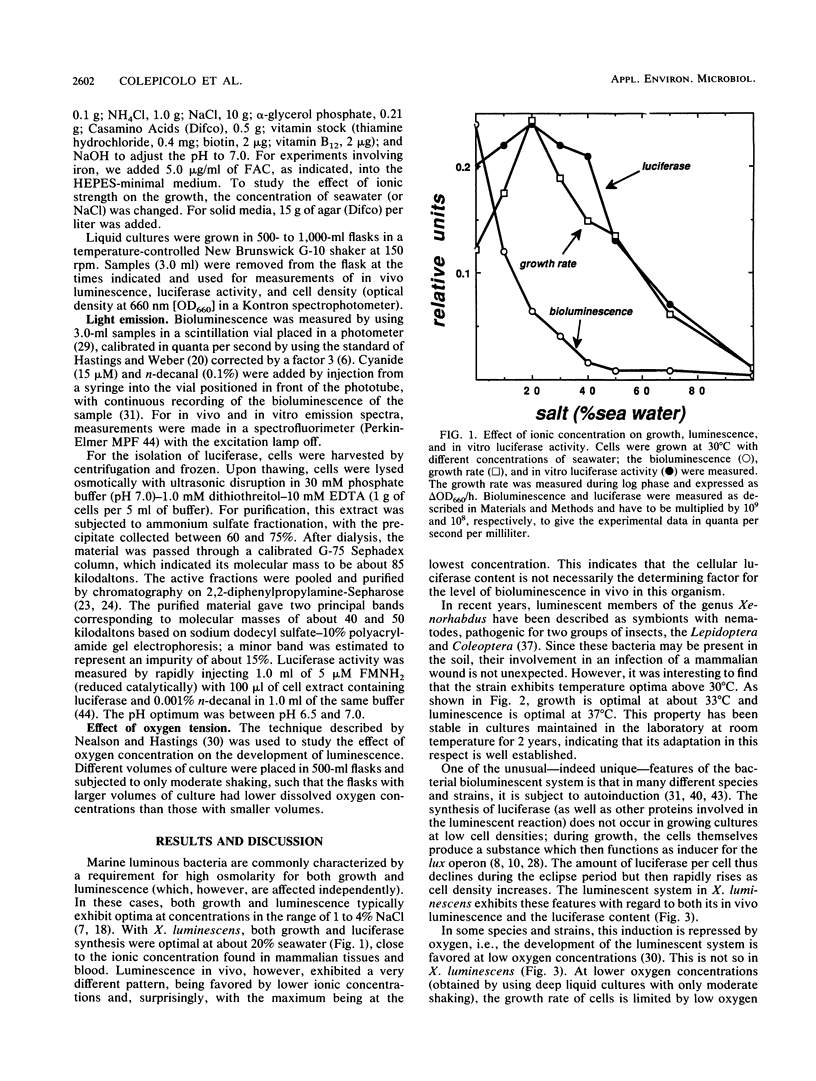
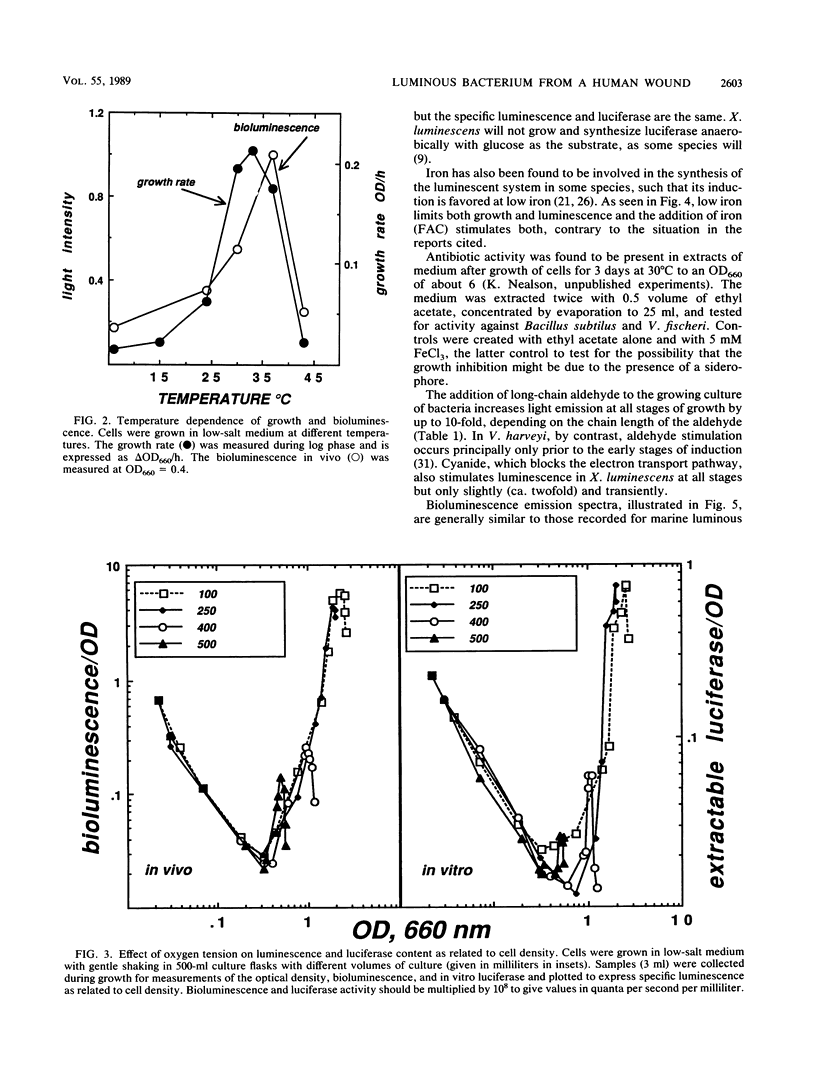
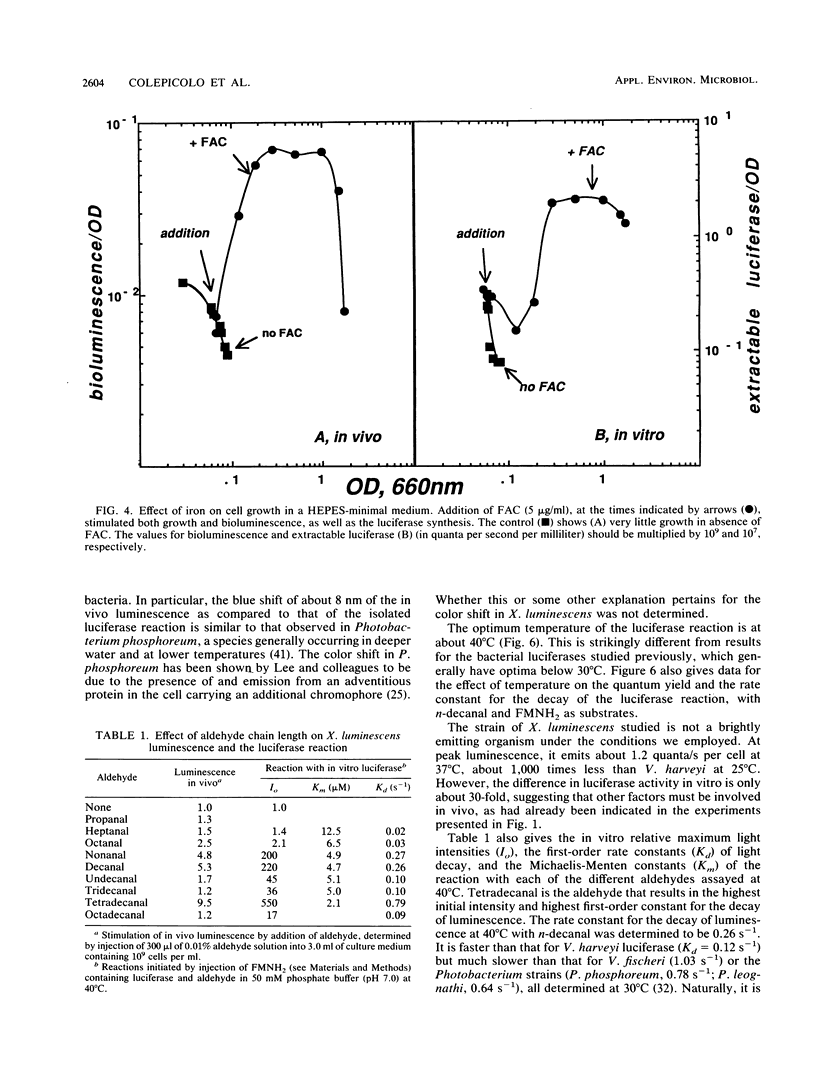
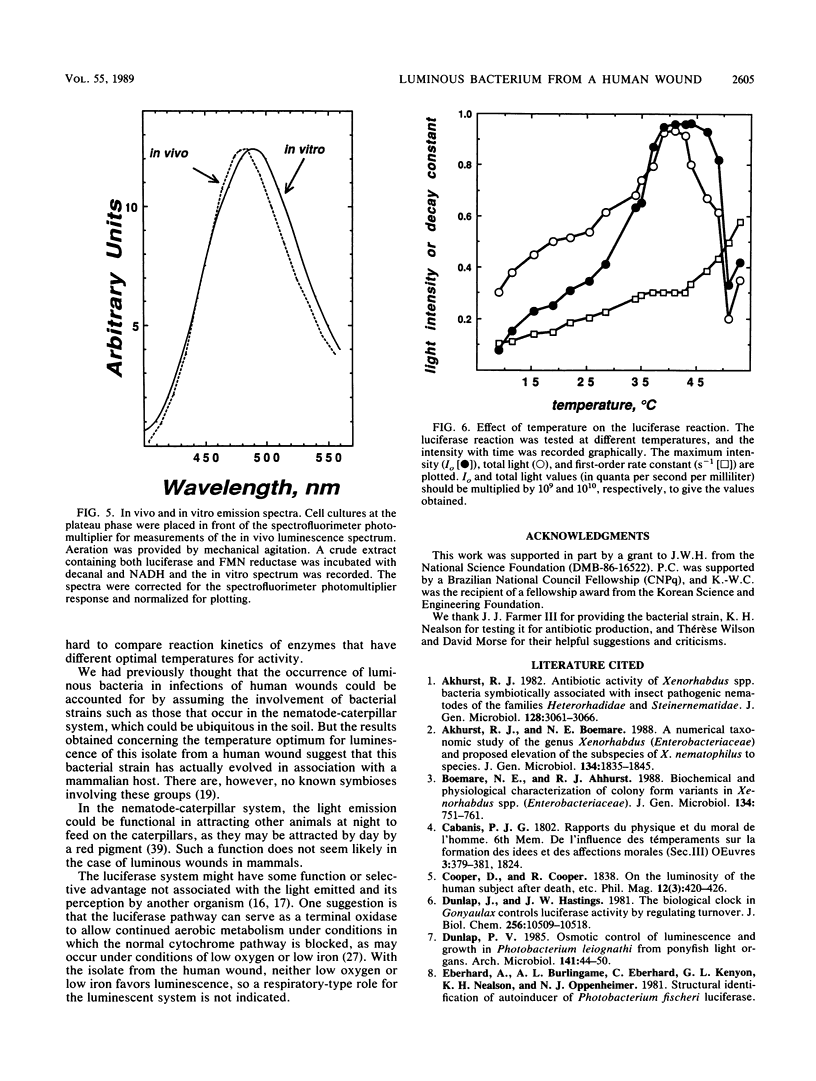
Xenorhabdus luminescens, a newly isolated luminous bacterium collected from a human wound, was characterized. The effects of ionic strength, temperature, oxygen, and iron on growth and development of the bioluminescent system were studied. The bacteria grew and emitted light best at 33 degrees C in a medium with low salt, and the medium after growth of cells to a high density was found to have antibiotic activity. The emission spectrum peaked at 482 nm in vivo and at 490 nm in vitro. Both growth and the development of luminescence in X. luminescens required oxygen and iron. The isolated luciferase itself exhibited a temperature optimum at about 40 degrees C; after purification by affinity chromatography, it showed two bands (52 and 41 kilodaltons) on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, indicative of an alpha and beta subunit structure. Reduced flavin mononucleotide (Km of 1.4 microM) and tetradecanal (Km of 2.1 microM) were the best substrates for the luciferase, and the first-order decay constant under these conditions at 37 degrees C was 0.79 s-1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982 Dec;128(12):3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Akhurst R. J., Boemare N. E. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species. J Gen Microbiol. 1988 Jul;134(7):1835–1845. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- Dunlap J. C., Hastings J. W. The biological clock in Gonyaulax controls luciferase activity by regulating turnover. J Biol Chem. 1981 Oct 25;256(20):10509–10518. [PubMed] [Google Scholar]
- Dunlap P. V. Osmotic control of luminescence and growth in Photobacterium leiognathi from ponyfish light organs. Arch Microbiol. 1985 Feb;141(1):44–50. doi: 10.1007/BF00446738. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Jorgensen J. H., Grimont P. A., Akhurst R. J., Poinar G. O., Jr, Ageron E., Pierce G. V., Smith J. A., Carter G. P., Wilson K. L. Xenorhabdus luminescens (DNA hybridization group 5) from human clinical specimens. J Clin Microbiol. 1989 Jul;27(7):1594–1600. doi: 10.1128/jcm.27.7.1594-1600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W. Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems. J Mol Evol. 1983;19(5):309–321. doi: 10.1007/BF02101634. [DOI] [PubMed] [Google Scholar]
- Holzman T. F., Baldwin T. O. Binding of 2,2-diphenylpropylamine at the aldehyde site of bacterial luciferase increases the affinity of the reduced riboflavin 5'-phosphate site. Biochemistry. 1981 Sep 15;20(19):5524–5528. doi: 10.1021/bi00522a027. [DOI] [PubMed] [Google Scholar]
- Holzman T. F., Baldwin T. O. Isolation of bacterial luciferases by affinity chromatography on 2,2-diphenylpropylamine-Sepharose: phosphate-mediated binding to an immobilized substrate analogue. Biochemistry. 1982 Nov 23;21(24):6194–6201. doi: 10.1021/bi00267a026. [DOI] [PubMed] [Google Scholar]
- Mitchell G. W., Hastings J. W. A stable, inexpensive, solid-state photomultiplier photometer. Anal Biochem. 1971 Jan;39(1):243–250. doi: 10.1016/0003-2697(71)90481-7. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Hastings J. W. Low oxygen is optimal for luciferase synthesis in some bacteria. Ecological implications. Arch Microbiol. 1977 Feb 4;112(1):9–16. doi: 10.1007/BF00446648. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Roberts D. M., White V. K., Dry M. A., Simpson L. M. Bioluminescence in a strain of the human pathogenic bacterium Vibrio vulnificus. Appl Environ Microbiol. 1986 Nov;52(5):1209–1211. doi: 10.1128/aem.52.5.1209-1211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. H., Schmidt T. M., Nealson K. H. Identification of an anthraquinone pigment and a hydroxystilbene antibiotic from Xenorhabdus luminescens. Appl Environ Microbiol. 1988 Jun;54(6):1602–1605. doi: 10.1128/aem.54.6.1602-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Greenberg E. P., Hastings J. W. Planktonic marine luminous bacteria: species distribution in the water column. Appl Environ Microbiol. 1980 Feb;39(2):302–306. doi: 10.1128/aem.39.2.302-306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters C. A., Hastings J. W. Mutants of luminous bacteria with an altered control of luciferase synthesis. J Bacteriol. 1977 Aug;131(2):519–525. doi: 10.1128/jb.131.2.519-525.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


