Abstract
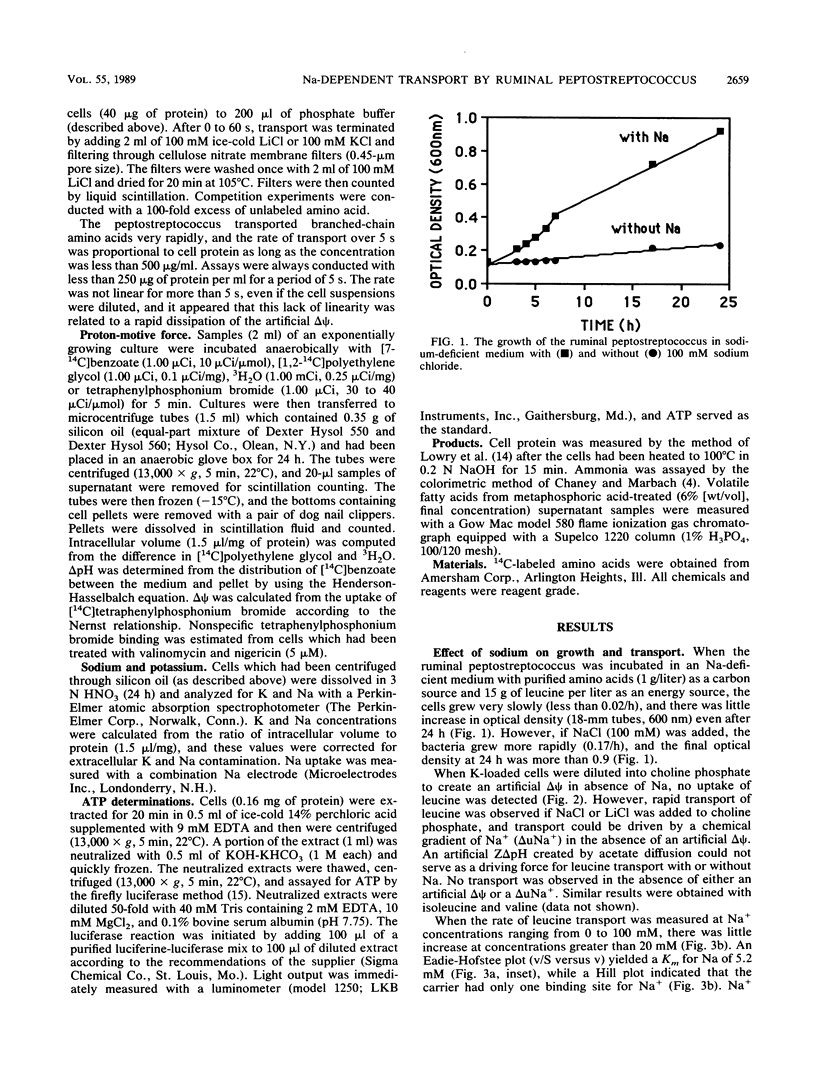
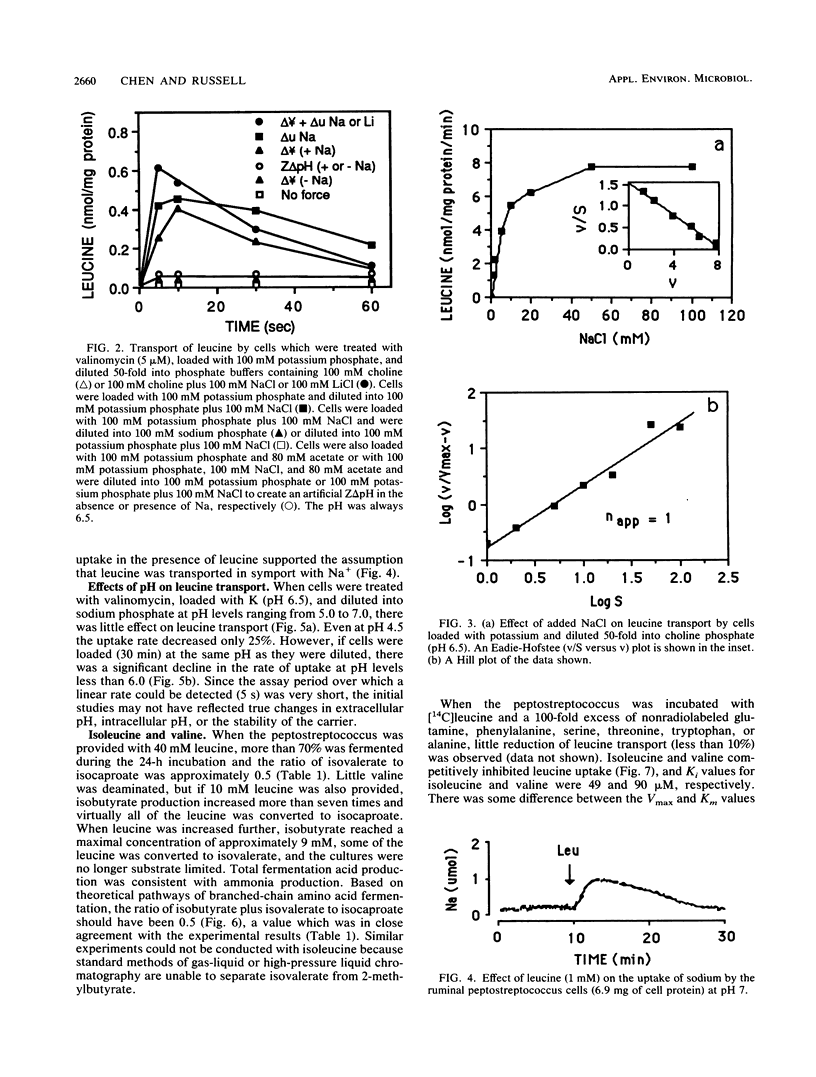
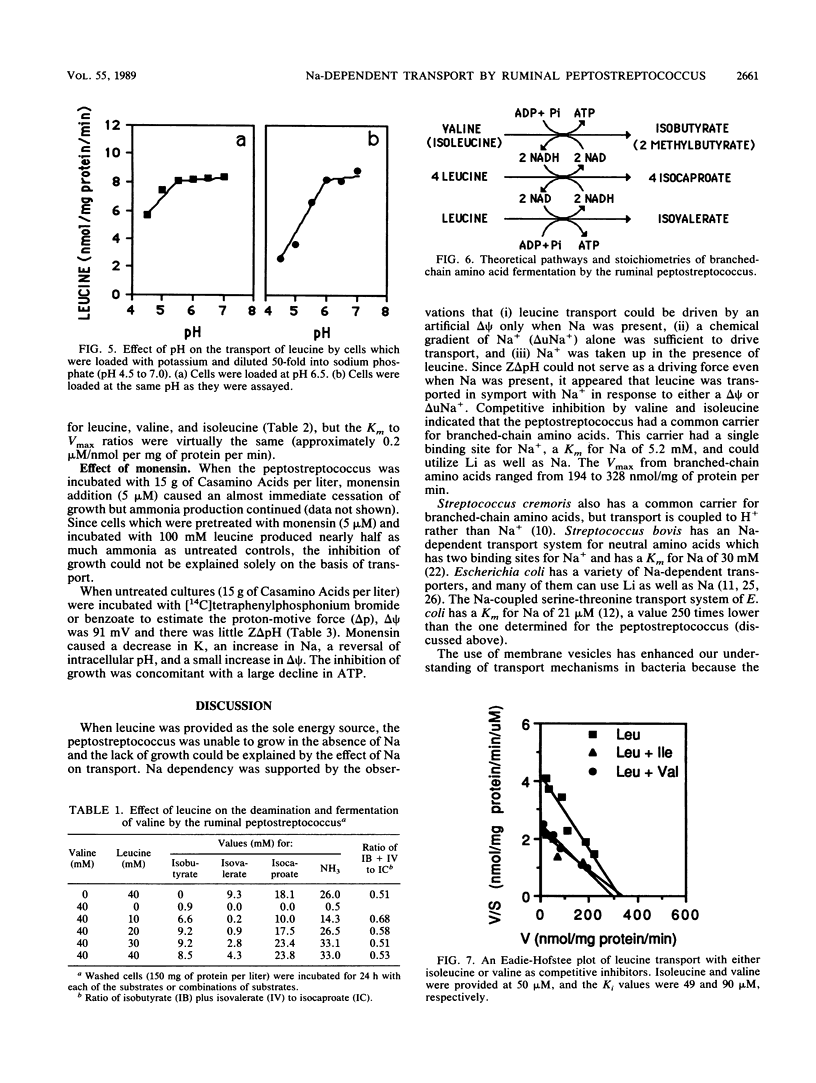
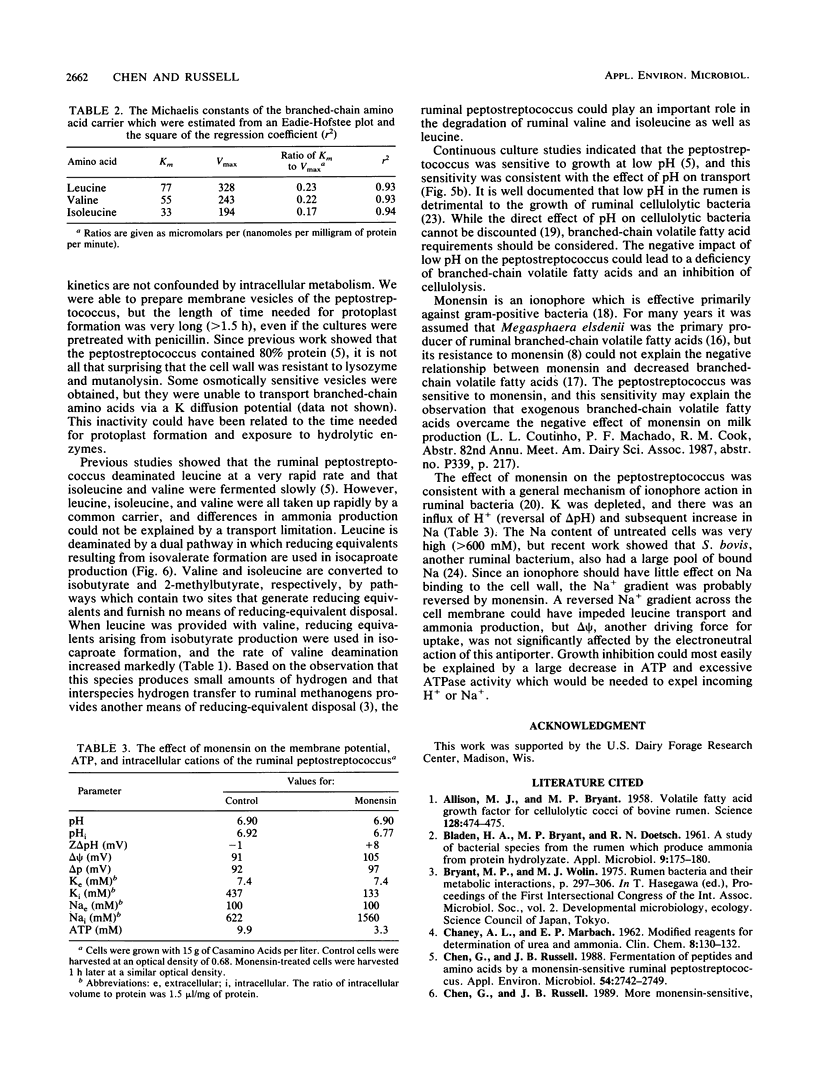
A recently isolated ruminal peptostreptococcus which produced large amounts of branched-chain volatile fatty acids grew rapidly with leucine as an energy source in the presence but not the absence of Na. Leucine transport could be driven by an artificial membrane potential (delta psi) only when Na was available, and a chemical gradient of Na+ (delta uNa+) also drove uptake. Because Na+ was taken up with leucine and a Z delta pH could not serve as a driving force (with or without Na), it appeared that leucine was transported in symport with Na+. The leucine carrier could use Li as well as Na and had a single binding site for Na+. The Km for Na was 5.2 mM, and the Km and Vmax for leucine were 77 microM and 328 nmol/mg of protein per min, respectively. Since valine and isoleucine competitively inhibited (Kis of 90 and 49 microM, respectively) leucine transport, it appeared that the peptostreptococcus used a common carrier for branched-chain amino acids. Valine or isoleucine was taken up rapidly, but little ammonia was produced if they were provided individually. The lack of ammonia could be explained by an accumulation of reducing equivalents. The ionophore, monensin, inhibited growth, but leucine was taken up and deaminated at a slow rate. Monensin caused a loss of K, an increase in Na, a slight increase in delta psi, and a decrease in intracellular pH. The inhibition of growth was consistent with a large decrease in ATP.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., DOETSCH R. N. Volatile fatty acid growth factor for cellulolytic cocci of bovine rumen. Science. 1958 Aug 29;128(3322):474–475. doi: 10.1126/science.128.3322.474. [DOI] [PubMed] [Google Scholar]
- Bladen H. A., Bryant M. P., Doetsch R. N. A Study of Bacterial Species from the Rumen Which Produce Ammonia from Protein Hydrolyzate. Appl Microbiol. 1961 Mar;9(2):175–180. doi: 10.1128/am.9.2.175-180.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Chen G. J., Russell J. B. Fermentation of peptides and amino acids by a monensin-sensitive ruminal Peptostreptococcus. Appl Environ Microbiol. 1988 Nov;54(11):2742–2749. doi: 10.1128/aem.54.11.2742-2749.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Russell J. B. More monensin-sensitive, ammonia-producing bacteria from the rumen. Appl Environ Microbiol. 1989 May;55(5):1052–1057. doi: 10.1128/aem.55.5.1052-1057.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S. M., Nagaraja T. G., Bartley E. E. Effects of lasalocid or monensin on lactate-producing or -using rumen bacteria. J Anim Sci. 1981 Feb;52(2):418–426. doi: 10.2527/jas1981.522418x. [DOI] [PubMed] [Google Scholar]
- Donius D. A., Simpson M. E., Marsh P. B. Effect of monensin fed with forage on digestion and the ruminal ecosystem of steers. J Anim Sci. 1976 Jan;42(1):229–234. doi: 10.2527/jas1976.421229x. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Jong S., Konings W. N. Transport of branched-chain amino acids in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1987 Nov;169(11):5193–5200. doi: 10.1128/jb.169.11.5193-5200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Yamato I., Anraku Y. Mechanism of glutamate transport in Escherichia coli B. 2. Kinetics of glutamate transport driven by artificially imposed proton and sodium ion gradients across the cytoplasmic membrane. Biochemistry. 1983 Apr 12;22(8):1959–1965. doi: 10.1021/bi00277a034. [DOI] [PubMed] [Google Scholar]
- Hama H., Shimamoto T., Tsuda M., Tsuchiya T. Properties of a Na+-coupled serine-threonine transport system in Escherichia coli. Biochim Biophys Acta. 1987 Dec 11;905(2):231–239. doi: 10.1016/0005-2736(87)90451-2. [DOI] [PubMed] [Google Scholar]
- Hino T., Russell J. B. Effect of reducing-equivalent disposal and NADH/NAD on deamination of amino acids by intact rumen microorganisms and their cell extracts. Appl Environ Microbiol. 1985 Dec;50(6):1368–1374. doi: 10.1128/aem.50.6.1368-1374.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H., Horiguchi M., Matsumoto T. Nutritional Interdependence Among Rumen Bacteria, Bacteroides amylophilus, Megasphaera elsdenii, and Ruminococcus albus. Appl Environ Microbiol. 1980 Aug;40(2):294–300. doi: 10.1128/aem.40.2.294-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. A proposed mechanism of monensin action in inhibiting ruminal bacterial growth: effects on ion flux and protonmotive force. J Anim Sci. 1987 May;64(5):1519–1525. doi: 10.2527/jas1987.6451519x. [DOI] [PubMed] [Google Scholar]
- Russell J. B. Effect of extracellular pH on growth and proton motive force of Bacteroides succinogenes, a cellulolytic ruminal bacterium. Appl Environ Microbiol. 1987 Oct;53(10):2379–2383. doi: 10.1128/aem.53.10.2379-2383.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Chen G. J. Enrichment and isolation of a ruminal bacterium with a very high specific activity of ammonia production. Appl Environ Microbiol. 1988 Apr;54(4):872–877. doi: 10.1128/aem.54.4.872-877.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. Effect of ionophores on ruminal fermentation. Appl Environ Microbiol. 1989 Jan;55(1):1–6. doi: 10.1128/aem.55.1.1-6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. S. Factors affecting the cellulolytic activity of rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):497–502. doi: 10.1128/aem.33.3.497-502.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel H. J., Russell J. B. Non-proton-motive-force-dependent sodium efflux from the ruminal bacterium Streptococcus bovis: bound versus free pools. Appl Environ Microbiol. 1989 Oct;55(10):2664–2668. doi: 10.1128/aem.55.10.2664-2668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Oho M., Shiota-Niiya S. Lithium ion-sugar cotransport via the melibiose transport system in Escherichia coli. Measurement of Li+ transport and specificity. J Biol Chem. 1983 Nov 10;258(21):12765–12767. [PubMed] [Google Scholar]
- Tsuchiya T., Yamane Y., Shiota S., Kawasaki T. Cotransport of proline and Li+ in Escherichia coli. FEBS Lett. 1984 Mar 26;168(2):327–330. doi: 10.1016/0014-5793(84)80272-0. [DOI] [PubMed] [Google Scholar]
- Van Nevel C. J., Demeyer D. I. Effect of monensin on rumen metabolism in vitro. Appl Environ Microbiol. 1977 Sep;34(3):251–257. doi: 10.1128/aem.34.3.251-257.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



