Abstract
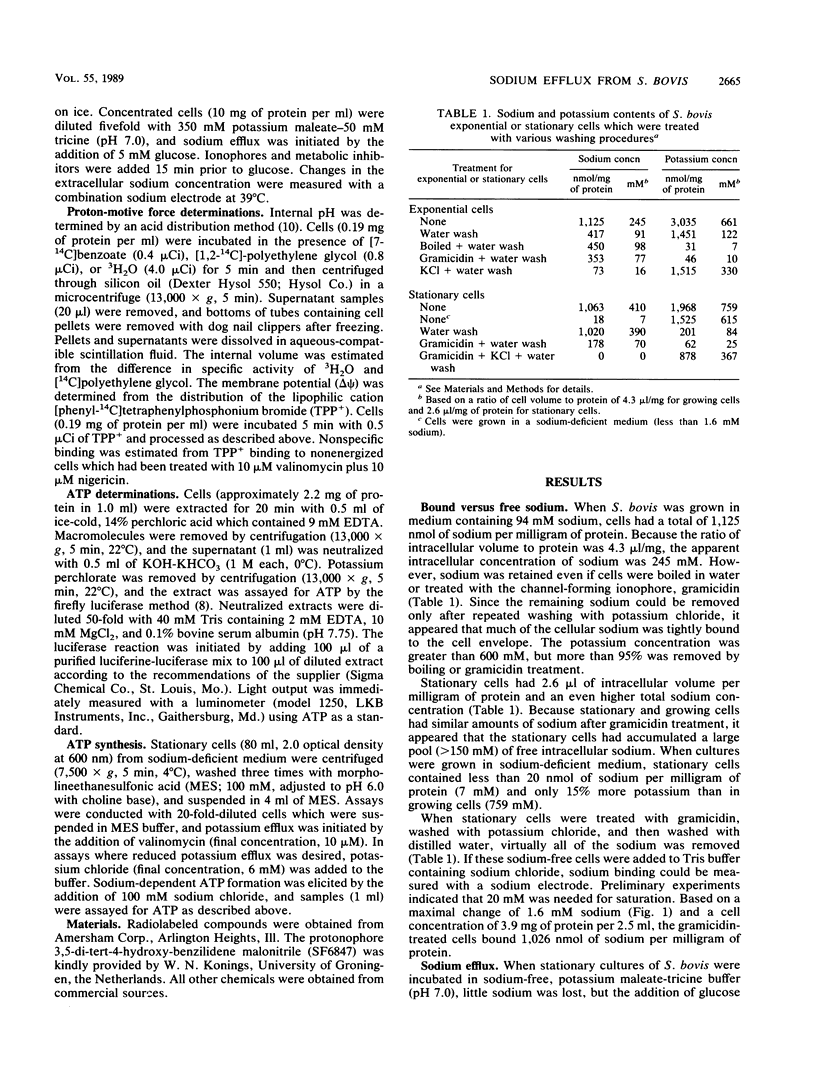
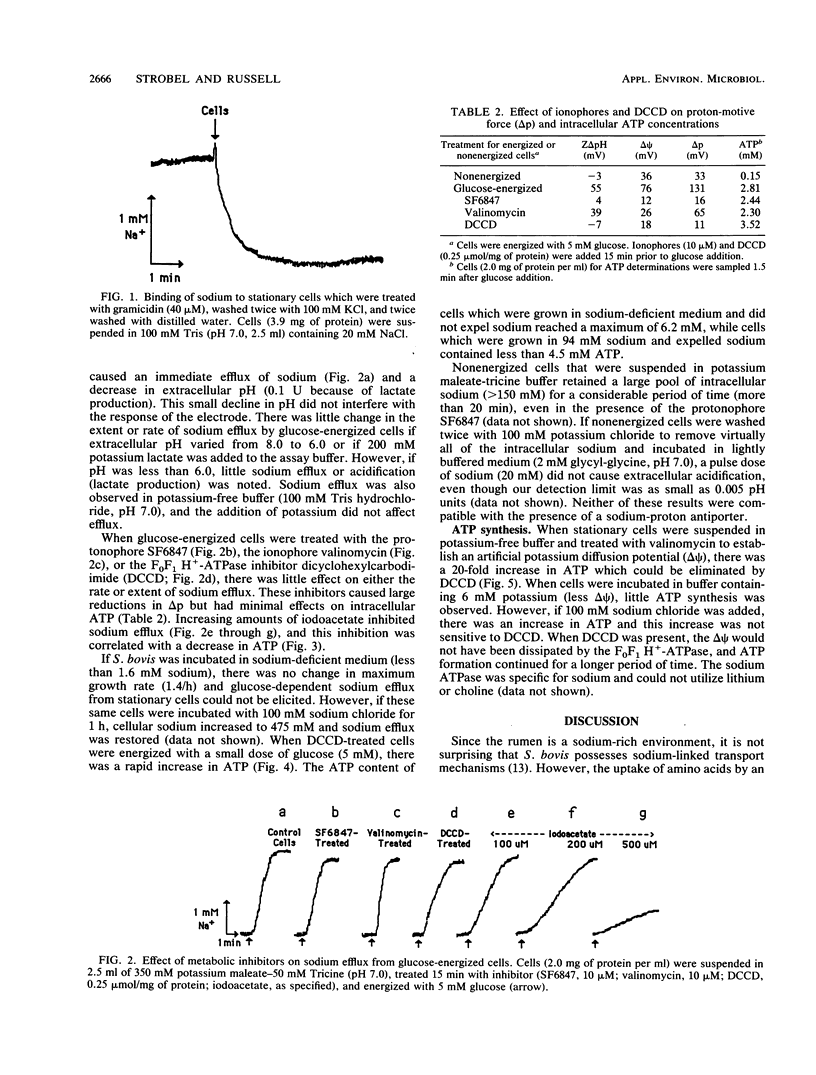
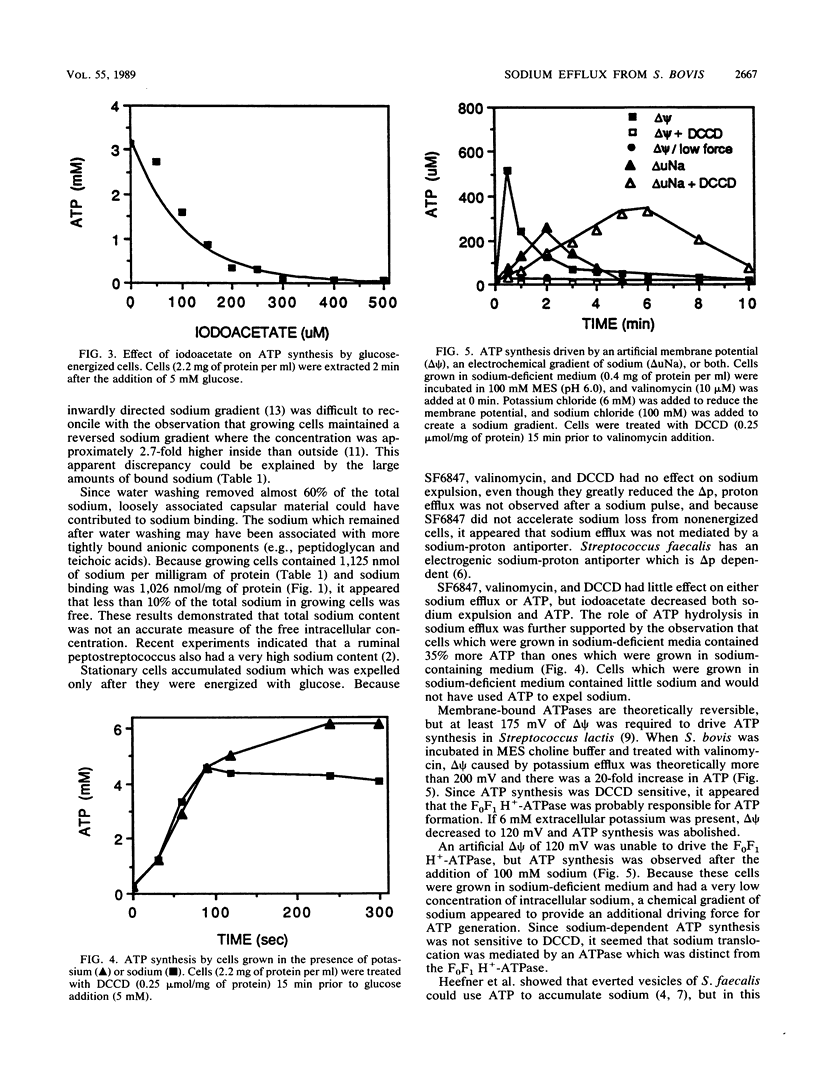
Growing cells of Streptococcus bovis JB1 had a sodium content of 1,125 nmol/mg of protein and, based on a ratio of cell volume to protein of 4.3 microliters/mg, the apparent intracellular sodium concentration was more than 240 mM. Much of this sodium could not be removed by water washing even if cells were boiled or treated with the pore-forming ionophore, gramicidin, but it could be exchanged for potassium. Stationary cultures had a 2.6-microliters volume per milligram of protein and a total sodium content of 410 mM. When stationary cultures were energized with glucose at pH 6 to 8, sodium (more than 200 mM) was expelled within 2 min, and it appeared that growing cells had a very small pool of free intracellular sodium. Sodium-proton antiport activity could not be demonstrated with a sodium pulse, and the protonophore SF6847, valinomycin, and the H+-ATPase inhibitor dicyclohexylcarbodiimide (DCCD) had little effect on sodium efflux, even though these inhibitors greatly reduced the proton-motive force. SF6847, valinomycin, and DCCD had little effect on intracellular ATP, but iodoacetate, an inhibitor of glycolysis, decreased ATP as well as sodium efflux. Stationary cells from sodium-deficient medium expelled little sodium after glucose addition and had 35% more ATP than stationary cells which were grown in sodium medium and expelled sodium. An artificial electrochemical gradient of sodium was able to drive ATP synthesis in stationary cells, and this ATP formation was not sensitive to DCCD. These results indicated that bacteria could have a significant pool of bound sodium and that sodium expulsion from S. bovis was directly coupled to ATP hydrolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell D. R., Hudson R. F. Sodium, an obligate growth requirement for predominant rumen bacteria. Appl Microbiol. 1974 Mar;27(3):549–552. doi: 10.1128/am.27.3.549-552.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. J., Russell J. B. Sodium-dependent transport of branched-chain amino acids by a monensin-sensitive ruminal peptostreptococcus. Appl Environ Microbiol. 1989 Oct;55(10):2658–2663. doi: 10.1128/aem.55.10.2658-2663.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heefner D. L., Kobayashi H., Harold F. M. ATP-linked sodium transport in Streptococcus faecalis. II. Energy coupling in everted membrane vesicles. J Biol Chem. 1980 Dec 10;255(23):11403–11407. [PubMed] [Google Scholar]
- Hilpert W., Schink B., Dimroth P. Life by a new decarboxylation-dependent energy conservation mechanism with Na as coupling ion. EMBO J. 1984 Aug;3(8):1665–1670. doi: 10.1002/j.1460-2075.1984.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma Y., Harold F. M. ATP-driven exchange of Na+ and K+ ions by Streptococcus faecalis. J Biol Chem. 1985 Feb 25;260(4):2086–2091. [PubMed] [Google Scholar]
- Kakinuma Y. Sodium/proton antiporter in Streptococcus faecalis. J Bacteriol. 1987 Sep;169(9):3886–3890. doi: 10.1128/jb.169.9.3886-3890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebeling V., Thauer R. K., Jungermann K. The internal-alkaline pH gradient, sensitive to uncoupler and ATPase inhibitor, in growing Clostridium pasteurianum. Eur J Biochem. 1975 Jul 1;55(2):445–453. doi: 10.1111/j.1432-1033.1975.tb02181.x. [DOI] [PubMed] [Google Scholar]
- Russell J. B. A proposed mechanism of monensin action in inhibiting ruminal bacterial growth: effects on ion flux and protonmotive force. J Anim Sci. 1987 May;64(5):1519–1525. doi: 10.2527/jas1987.6451519x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Robinson P. H. Compositions and characteristics of strains of Streptococcus bovis. J Dairy Sci. 1984 Jul;67(7):1525–1531. doi: 10.3168/jds.S0022-0302(84)81471-X. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



