Abstract
Background
γδ T cells have an important immunoregulatory and effector function through cytokine release. They are involved in the responses to Gram-negative bacterium and in protection of lung epithelium integrity. On the other hand, they have been implicated in airway inflammation.
Methods
The aim of the present work was to study intracytoplasmic IL-2, IL-4, IFN-γ and TNF-α production by γδ and αβ T lymphocytes from cystic fibrosis patients and healthy donors in response to Pseudomonas aeruginosa (PA). Flow cytometric detection was performed after peripheral blood mononuclear cells (PBMC) culture with a cytosolic extract from PA and restimulation with phorbol ester plus ionomycine. Proliferative responses, activation markers and receptor usage of γδ T cells were also evaluated.
Results
The highest production of cytokine was of TNF-α and IFN-γ, γδ being better producers than αβ. No differences were found between patients and controls. The Vγ9δ2 subset of γδ T cells was preferentially expanded. CD25 and CD45RO expression by the αβ T subset and PBMC proliferative response to PA were defective in cystic fibrosis lymphocytes.
Conclusion
Our results support the hypothesis that γδ T lymphocytes play an important role in the immune response to PA and in the chronic inflammatory lung reaction in cystic fibrosis patients. They do not confirm the involvement of a supressed Th1 cytokine response in the pathogenesis of this disease.
Keywords: cystic fibrosis, cytokines, Pseudomonas aeruginosa, γδ T lymphocytes.
Introduction
Cystic Fibrosis (CF) is the most common serious recessively inherited disease among Caucasians [1]. It is associated with impaired mucociliary clearance, abnormally thick mucus, chronic infections and lung inflammation. Most of CF patients are chronically colonized by bacteria such as Pseudomonas aeruginosa (PA) in their respiratory tract, this microorganism being their main cause of morbidity and mortality [2]. A single major mutation (ΔF508) in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene is responsible for 70% of CF cases, but over 800 mutations have been identified. CFTR is an anion channel and it negatively regulates the amiloride-sensitive epithelial Na channel [3]. Individuals, however, with mutations that affect apical epithelial Na+ channel function do not have infectious lung disease. In primary ciliary diskinesia, the absence of mucociliary clearance results in recurrent lung infections, but the diagnosis is often delayed until adulthood and PA colonization is absent. All this evidence suggests that other factors are involved in CF.
CFTR was found in CD4-positive T cells and a Cl- channel function, similar to that regulated by CFTR in epithelial cells, was detected in these lymphocytes. This function was defective in CF patients [4]. Therefore CFTR mutations could affect immunocompetent or accessory cells. In fact immunoregulatory defects in CF patients have been described and include reduced supressor and helper T cell activity [5].
Most of T cells bear the heterodimeric αβ antigen receptor but a relatively small subset express two different rearranging genes, γ and δ. About 5% of human peripheral blood lymphocytes are γδ T lymphocytes, but they are preferentially localized in epithelial and mucosal tissues. Protective response to pulmonary injury requires γδ T lymphocytes [6,7]. In previous reports we described an increased percentage of γδ T cells in peripheral blood of CF patients [8] and we showed, in healthy individuals, a preferential "in vitro" proliferation of these cells in response to PA cytosolic non-peptidic phosphorilated antigens [9]. γδ T cells have been described as an immunoregulatory subset [10,11] and their cytotoxic activity has been associated with some diseases [12,13]. Specific changes in γδ T receptor genes usage related to function have been described in patients with pulmonary tuberculosis [14].
In the last few years attention has been focused on the role of cell-mediated immunity in host defense against bronchopulmonary infection with Gram-negative bacterium. A correlation between poor lung function and elevated bacteria-specific antibody level in CF patients and animal models was demonstrated [15]. Furthermore, protective immune responses in animal models mimicking CF were cell-mediated [15-17]. The mechanisms by which T cells protect the lung are not completely known but it has been demonstrated that they activate the local phagocytic response through cytokine release [18]. γδ T cells have been described as important inducers of TNF-α production by LPS-stimulated macrophages [19]. In addition, mice depleted of these lymphocytes showed exaggerated bacterial growth after E. coli infection [20]. Recent "in vivo" experiments have demonstrated that T lymphocytes expressing Vγ9 and Vδ2 genes are mediators of resistance against extracellular gram-negative and positive bacteria [21].
PA has a biofilm mode of growth that could be regarded as a protective multicellular survival strategy, resembling intracellular bacteria such as mycobacteria. Th1-type cytokines, as IFN-γ and TNF-α, are responsible for a good immune response to intracellular bacteria; in contrast Th2-type cytokines, as IL-4, induce antibody production by B lymphocytes [22]. Production of Th1 cytokines by CD4-positive lymphocytes has been suggested to be impaired in CF [17,23].
γδ T cells are a Th1-type cytokines source [24,25] and their role in the "in vivo" mycobacteria clearance in macaques has been recently demonstrated [26]. A defect in γδ T lymphocytes activation or a bias in their cytokine secretion in response to PA could contribute to the chronic colonization by this bacterium. Alternatively chronic γδ T cells activation could determine a local persistent inflammation in the lung.
The aim of the current work was to assess the "in vitro" response of αβ and γδ T lymphocytes from peripheral blood of healthy controls and CF patients to cytosolic antigens from PA. To estimate this response we evaluated two surface markers: CD25, that corresponds to the IL-2 receptor α-chain and CD45RO, a putative memory marker. CD25 expression by T lymphocytes correlates with proliferative responses to antigens and mitogens and has been described as more sensitive than others markers, as CD69, to minor subpopulations responses [27]. CD45RO is expressed by T cells after antigen contact and further antigenic stimulus, at tissue level, commit CD45RO-positive cells to an effector function [28]. This marker is then expressed by both memory and effector T cells.
To assess the type of cytokines secreted in response to PA, intracytoplasmic IL-4, IFN-γ, TNF-α and IL-2 production from αβ and γδ T cells was determined by three color flow cytometry. Phenotypic characteristics of the expanded γδ T subpopulation and PBMC proliferative activity were also evaluated.
Materials and Methods
Donors
14 clinically stable outpatients with Cystic fibrosis (8 males and 6 females), age range: 8–29 years (CFw group) and 17 sex/age matched healthy controls, were studied. All CF patients had diagnosis confirmed by pilocarpine iontophoresis sweat test; 5 patients were homozygous for the ΔF508 mutation and the remainder were heterozygous for this mutation.
Patients were not receiving systemic corticosteroids and their treatment included pancreatic enzymes, vitamins and, in some cases, antibiotic therapy and sympathicomimetics. Patients chronically colonized by PA received cycles of an antipseudomonal penicillin and an aminoglycoside, oral or intravenous, depending on the antibiogram. Two DMID cases were on insulin therapy.
The clinical severity of the disease was estimated using the Schwachman score (SC). A SC from 41 to 55 corresponds to moderate severity, from 56 to 70 to slight severity, from 71 to 85 to good status and from 86 to 100 to excellent status. In our patients, SC ranged from 55 to 95 (Table 1).
Table 1.
Characteristics of cystic fibrosis patients included in the study
| Patients | Age | Mutations | History of infections | PA culture | Shwachman score | Additional Data |
| MGV | 15 | ΔF508/ΔF508 | PA chronic colonization | Pos | 95 | |
| MGP | 14 | ΔF508/ΔF508 | PA chronic colonization, occasional SA | Pos | 76 | |
| AAS | 17 | ΔF508/? | PA chronic colonization | Pos | 60 | |
| AGH | 15 | ΔF508/? | PA chronic colonization | Pos | 80 | |
| MCC | 14 | ΔF508/? | SA and PA chronic colonization | Pos | 65 | DMID |
| LGS | 5 | ΔF508/ΔF508 | PA chronic colonization | Pos | 85 | |
| EML | 29 | R347H/ΔF508 | PA chronic colonization | Pos | 55 | |
| DNP | 12 | ΔF508/2789+ 563A | PA chronic colonization | Pos | 95 | |
| YML | 23 | R347H/ΔF508 | PA chronic colonization | Pos | 55 | |
| MVG | 8 | ΔF508/ΔF508 | occasional SA | Neg | 90 | |
| VMM | 8 | ΔF508/G542X | # | Neg | 85 | |
| ASA | 7 | ΔF508/L206W | SA chronic colonization, occasional HI | Neg | 95 | |
| GCF | 10 | ΔF508/ΔF508 | occasional SA | Neg | 85 | |
| MSP | 21 | ΔF508/? | * PA chronic colonization | * | 71 | DMID |
PA: Pseudomonas aeruginosa, SA: Staphylococcus aureus, HI: Haemophilus influenzae. Pos: sputum culture positive for PA, Neg: sputum culture negative for PA, DMID:Diabetes mellitus insulin-dependent. # PA colonization until 2 years before blood extraction, negative from this date until now * PA negative only for one year, blood extraction was made during this year Schwachman score rates severity based on history, physical examination and chest roentgenogram
PA was detected, by sputum culture, in 9 patients, all of them chronically colonized by PA (CFp group). Four patients were free of PA infection at the moment of the study (CFn group), 3 of these have always been free of PA and 1 was colonized by PA until two years before the study and from this date he has been PA-negative. One patient has always been PA-positive except for a year that included the blood extraction data. This last patient was included in the whole (CFw) group but not in the CFp or in the CFn group. Genetic, clinical and infectious characteristics of the patients are depicted in Table 1.
The study was approved by the Hospital ethical committee and informed consent was obtained from participants.
Activation kinetics and phenotypic studies
Heparinized peripheral blood was obtained from patients and controls. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque and cultured in RPMI-1640 supplemented with 10% heat-inactivated autologous serum, at 37°C, 5% CO2 in air. Heat-treated cytosolic fraction from PA (PAc) was obtained from a sputum isolated strain as already described [9]. Briefly, we cultured the isolated bacteria in liquid media and then washed and heat-treated them at 120°C for 20 min. Lysates were prepared by passage through a French press and the supernatants obtained after centrifugation (7840 × g). Cytosolic antigens (PAc), that were the main PA stimulatory fraction to γδ T cells [9], were obtained by lysates ultracentrifugation (80000 × g for 45 min). PAc was added to PBMC cultures at 50 μg/ml.
CD25 and CD45RO expression by αβ and γδ T cells was evaluated by three-color flow cytometry before and after 4, 6 and 8 days of culture. We chose these days of culture because T lymphocytes stimulated with antigen or mitogen show a peak of CD25 and CD45RO expression between 4 and 8 days of culture [27,29].
Cells were acquired and analysed on a FACScalibur cytometer, using CellQuest software, Becton-Dickinson, Mountain View, CA, USA (BD). Anti-CD3-PerCP, anti-TCR αβ-FITC and anti-CD25 or anti-CD45RO-PE MAbs (BD) were used.
In a previous work we demonstrated a preferent γδ T subset expansion between 7 and 10 days of PBMC culture with a PA cytosolic extract [9]. In the current study γδ T cells phenotype was determined, by three-color flow cytometry, before and after 8 days of culture. 2500 γδ T cells were acquired and analysed in dot-plot cytograms. Anti-TCRγδ-PE from Immunotech (Marseille. France), anti-CD8-PerCP and anti-CD4-FITC (BD) or either anti-Vγ9 or anti-Vδ2-FITC from Immunotech were used.
PBMC Proliferation
PBMC (2 × 105 / well) from controls and patients were cultured with PAc (50 μg/ml) or Phytohemaglutinin (PHA) at 1 μg/ml (GIBCO BRL. Eragny. France), in RPMI-1640 supplemented with 10% heat-inactivated autologous serum, for 6 and 3 days respectively. Cells were pulsed with 1 μCi [3H]-thymidine (Amersham, UK) for 16 h.
Stimulation index (SI) was calculated by the quotient between counts per minute (c.p.m.) obtained with and without stimulus.
Intracellular cytokine production in response to P. aeruginosa and restimulation with Phorbol 12-Myristate 13-Acetate plus Ionomicine
The activation of γδ and αβ T lymphocytes with PAc produced only low intracytoplasmic cytokine signals. Phorbol 12-Myristate 13-Acetate (PMA) plus Ionomicine (Io) has been described as non distorting, policlonal stimulus, for previously expanded antigen-specific T cells [30-32]. In the current work we determined the percentage of γδ and αβ cytokine-producing cells at day 10 of PBMC culture with PA cytosolic extract, after restimulation with PMA-Io. On this day, the γδ subset reached a maximal expansion without excessive cellular death. Measurement of single cell intracellular cytokines allowed us to determine the kind of produced cytokines and which T subpopulation was their source. This system avoided the purification of a minor T subset, as the γδ-positive, that could require an excessive amount of blood sample, not available from children patients.
PBMC in complete medium (2 × 106/well) were incubated with PAc (50 μg/ml) as above described. Cells were harvested on day 10, washed and left 23 h. in culture medium, without stimulus, at 106 cells/well. BFA (10 μg/ml), PMA (25 ng/ml) and Io (1 μg/ml) was added for 4 h. at 37°C, 5% CO2 in air. After washing, cells were incubated for 15 min at room temperature with MAbs: anti-TCRγδ-1-FITC (BD) or PE-conjugated (Immunotech) and anti-CD3-PerCP (BD), fixed with FACS Lysis Solution (BD) and washed. The pellet was incubated with 200 μl of FACS Permeabilizing Solution (BD) for 10 min, washed and incubated for 30 min. at room temperature with either the anti-cytokine monoclonal antibody: anti-IL-2, anti-IL-4-PE (BD), anti-TNF-α-PE (CALTAG. Burlingame, CA) or anti-IFN-γ-FITC from Serotec (Oxford, England). The cells were then washed and resuspended with 100 μl of 1% paraformaldehide.
Samples were acquired and analysed using CellQuest software (BD). Ten-thousand T lymphocytes were acquired according to CD3 expression. The percentage of cytokine-positive cells within each subset, γδ-positive or negative was calculated. Irrelevant, isotype-matched antibodies were used in parallel with all experimental samples.
Statistical analysis
For means comparison between two different groups and between paired samples, the two tailed Student t or the Mann-Whitney U test was used. For means comparison between three groups (Controls, CFp and CFn) oneway ANOVA and LSD PostHoc or the Kruskal-Wallis test were used. Data are given as mean ± standard error of the mean, p values are two-sided and considered significant when <0.05. The calculations were performed using SPSS 6.0 software.
Results
Activation kinetics and phenotypic studies
The percentage of γδ-positive T lymphocytes before PBMC culture was significantly higher in CFw (14.7 ± 2.4) than in controls (5.8 ± 0.9), p < 0.01. During culture with PAc γδ percentage first sligthly decreased, but from day 4 it progressively increased in all groups and there were no differences between groups (Fig 1).
Figure 1.
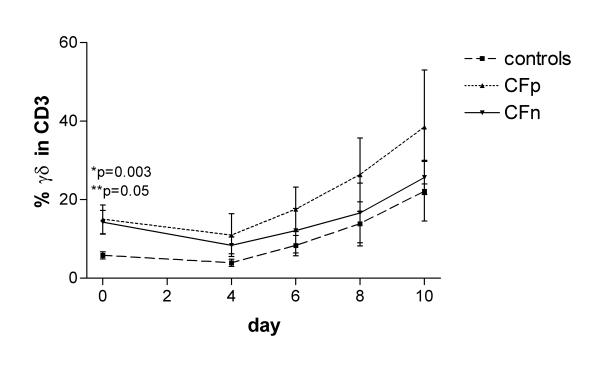
Percentage of γδ positive cells in CD3-positive lymphocytes after 0, 4, 6, 8 and 10 days of PBMC culture with cytosolic extract from Pseudomonas aeruginosa (PA). Surface expression of γδ receptor was detected in 2500 gated T lymphocytes, by three color cytometry, in controls (n = 17), Cystic fibrosis patients infected by PA (CFp, n = 9) and Cystic fibrosis patients not infected by PA (CFn, n = 4). Bar values correspond to mean ± standard error of the mean. (*) significant difference between controls and CFp, (**) significant difference between controls and CFn (ANOVA, LSD PostHoc test).
Most of γδ T lymphocytes were Vγ9Vδ2 before and after culture. The percentages of Vγ9+ and Vδ2+ cells were significantly higher at the end than at the beginning of culture in CFw, CFp and in controls (Table 2). CFn showed higher values of Vγ9+ and Vδ2+ at the end than before culture but without statistical significance, probably due to the low number of individuals. Before and during culture, few cells expressed CD8 and very few expressed CD4 in all groups. There were no significant differences in γδ T cells phenotype between patients and controls.
Table 2.
Phenotypic characteristics of γδ T cells before and after 8 days of culture with cytosolic extract from P. aeruginosa (PA)
| Vγ9 day 0 | Vγ9 day 8 | Vδ2 day 0 | Vδ2 day 8 | CD4 day 0 | CD4 day 8 | CD8 day 0 | CD8 day 8 | |
| Controls | 84.71 ± 3.12** | 92.85 ± 1.70 | 77.67 ± 3.8* | 89.46 ± 3.43 | 2.03 ± 0.93 | 0.76 ± 0.65 | 11.92 ± 2.55 | 10.13 ± 1.91 |
| CFw | 89.83 ± 2.78* | 94.75 ± 2.06 | 84.65 ± 3.89* | 92.39 ± 3.23 | 0.49 ± 0.26 | 0.27 ± 0.18 | 9.21 ± 1.17 | 10.13 ± 1.91 |
| CFp | 92.52 ± 1.40** | 96.82 ± 1.14 | 85.90 ± 3.88** | 94.44 ± 2.72 | 0.78 ± 0.37 | 0.54 ± 0.31 | 9.48 ± 1.80 | 9.84 ± 2.69 |
| CFn | 82.80 ± 8.8 | 87.45 ± 7.98 | 79.46 ± 10.32 | 87.32 ± 8.64 | 0.1 ± 0.01 | 0.1 ± 0.01 | 8.41 ± 1.53 | 11.28 ± 3.42 |
Results are expressed as the percentage of positive cells (mean ± s.e.m.) in the γδ T subpopulation. CFw (cystic fibrosis patients, n = 14), CFp (cystic fibrosis patients infected by PA, n = 8), CFn (cystic fibrosis patients without PA, n = 4). Means comparison between paired samples (days 0 and 8) were made by the Student's t test: *Significantly lower than the value at day 8: p < 0.01 **Significantly lower than the value at day 8: p < 0.05
The percentage of CD25-positive γδ T cells was negligible on day 0 but experienced an important increase after 4 days of culture, prior γδ expansion. From day 6 to 8 it reached a maximum, over 50% of cells. A higher percentage of CD25-positive αβ than γδ was found at the beginning of culture in controls (p < 0.001), CFp and CFn (p < 0.05), but the increase during the culture was higher in γδ T cells. In consequence the percentage of CD25-positive cells was higher in the γδ subset on days 6 (controls: p < 0.001, CFp: p = 0.001, CFn: not significant) and 8 (controls: p < 0.05, CFp: p < 0.001, CFn: not significant) (Fig 2) and corresponded to their preferent expansion.
Figure 2.
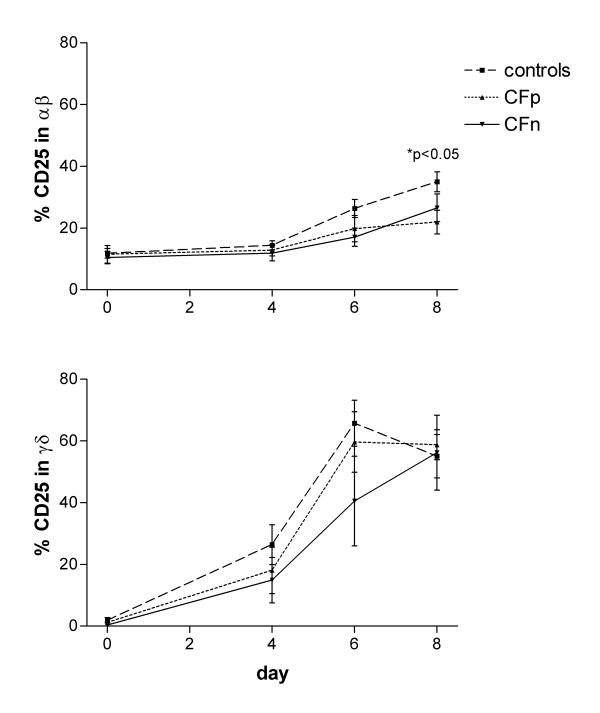
Percentage of CD25-expressing cells in αβ T lymphocytes (top) and γδ T lymphocytes (bottom), after 0, 4, 6 and 8 days of PBMC culture with cytosolic extract from Pseudomonas aeruginosa. Surface expression of CD25 (α chain of IL-2 receptor) was detected, by three color cytometry, in 2500 gated T lymphocytes from controls (n = 17), Cystic fibrosis patients infected by PA (CFp, n = 9) and Cystic fibrosis patients not infected by PA (CFn, n = 4). Bar values correspond to mean ± standard error of the mean. (top) αβ T lymphocytes: (*) significant difference between controls and CFp (ANOVA, LSD PostHoc test). (bottom) γδ T lymphocytes: significant differences were not found between groups.
The αβ CD25 expression at the end of culture (day 8) was higher in controls than in CFp (p < 0.05) and CFn (not significant), but there were no differences in the γδ CD25 expression between groups (Fig 2).
Before culture, the percentage of γδ CD45RO-positive cells was significantly higher in CFw (78.9 ± 3.2) than in controls (62.7 ± 5.6) (p < 0.05). When we separated patients into CFn and CFp groups, the difference was not significant (Fig 3). After day 4 there were no significant differences between CFw and controls, due to the increase in the control group. CD45RO expression was always significantly higher in γδ than in αβ, agreeing with other reports [33], in controls: p < 0.05 on day 0 and p < 0.001 during culture, in CFp: p < 0.001 on day 0 and during culture and in CFn: p < 0.05 on day 0 and during culture (Fig 3).
Figure 3.
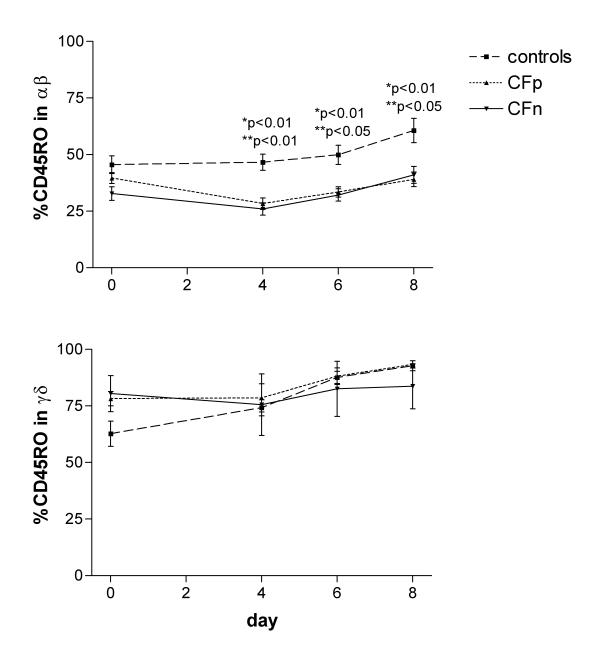
Percentage of CD45RO-expressing cells in αβ T lymphocytes (top) and γδ T lymphocytes (bottom), after 0, 4, 6, and 8 days of PBMC culture with cytosolic extract from Pseudomonas aeruginosa. Surface expression of CD45RO (memory/activation marker) was detected, by three color cytometry, in 2500 gated T lymphocytes from controls (n = 17), Cystic fibrosis patients infected by PA (CFp, n = 9) and Cystic fibrosis patients not infected by PA (CFn, n = 4). Bar values correspond to mean ± standard error of the mean. (top) αβ T lymphocytes: (*) significant difference between controls and CFp, (**) significant difference between controls and CFn (ANOVA, LSD PostHoc test). (bottom) γδ T lymphocytes: significant differences were not found between groups.
The percentage of αβ CD45RO-positive before culture was higher, but not significantly different in controls (45.54 ± 3.9) than in CFw (37.4 ± 2.06). Following stimulation with PAc it increased only in the former group. At day 8, controls had significantly higher results than CFp (p < 0.01) and CFn (p < 0.05).
PBMC Proliferation
Statistically significant differences between patients and controls were only found for SI in response to PA. They were higher in controls: 67.7 ± 37.8 than in CFw: 14 ± 5.4 (Mann-Whitney U test, p < 0.05). When patients were separated depending on PA infection, the differences were not significant (Fig 4).
Figure 4.
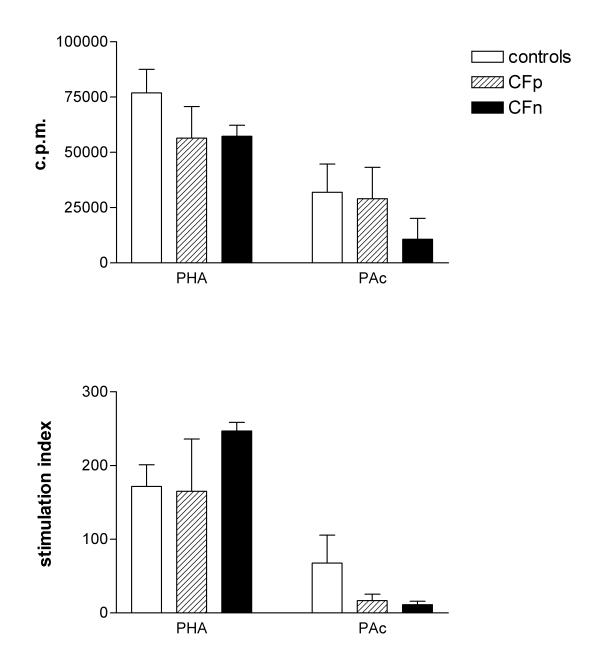
PBMC Proliferative response (3H-thymidine incorporation), expressed as c.p.m. (top) and stimulation index (bottom), after culture either with cytosolic extract from Pseudomonas aeruginosa (PAc) for 6 days or with Phytohemaglutinin (PHA) for 3 days. Significant differences were not found between controls (n = 10), Cystic fibrosis patients infected by PA (CFp, n = 9) and Cystic fibrosis patients not infected by PA (CFn, n = 4), (Kruskal-Wallis test) Bar values correspond to mean ± standard error of the mean.
Intracellular cytokine production in response to P. aeruginosa and PMA/Io restimulation
The frequency of cytokine-producing cells within both T-cell subsets was found in the order of TNF-α>IFN-γ>IL-2 (Fig 5). The percentage of IL-4-producing cells was in all cases negligible.
Figure 5.
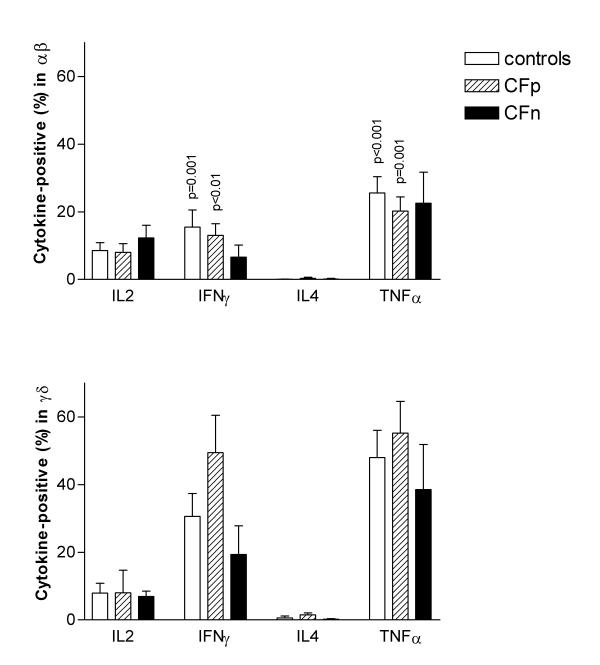
Percentage of intracytoplasmic cytokine-producing cells within αβ T cells (top) and γδ T cells (bottom) after 10 days of culture with cytosolic extract from Pseudomonas aeruginosa, followed by 4 hs restimulacion with PMA-Io. Intracytoplasmic cytokine expression was detected, by three color cytometry, in 10000 gated T lymphocytes. Column values correspond to mean, bar values correspond to standard error of the mean. Significant differences were not found between groups (ANOVA). p values were obtained from means comparison between αβ and γδ T lymphocytes within each group: controls (n = 17), Cystic fibrosis patients infected by PA (CFp, n = 9) and Cystic fibrosis patients not infected by PA (CFn, n = 4) (Student t test for paired samples).
There were no significant differences between controls and CFw, or between controls, CFp and CFn groups, but γδ T cells from CFp showed higher TNF-α and IFN-γ values than controls and CFn (Fig 5).
We detected higher IFN-γ production in γδ T cells, in comparison to αβ, in CFw, γδ: 36.9 ± 8.4; αβ: 10.3 ± 2.5 (p < 0.01) and in controls, γδ: 30.6 ± 6.7; αβ: 15.5 ± 5.1 (p = 0.001). The same was found for TNF-α in CFw, γδ: 47.4 ± 7.4; αβ: 25.5 ± 4.8 (p = 0.001) and in controls, γδ: 47.9 ± 8.1; αβ: 20.2 ± 3.7 (p < 0.001). When we considered CFp and CFn separately, the statistical significance only remained in CFp, however IFN-γ and TNF-α results were also higher in γδ than in αβ from CFn (Fig 5).
The percentage of cytokine-producing cells, αβ or γδ-positive, in total T lymphocytic population was calculated (Fig 6). Both subsets contributed to the same extent in the percentage of IFN-γ and TNF-α-positive lymphocytes. In contrast, the highest number of IL-2-producing lymphocytes always proceeded from the αβ subset. Again, we did not find significant differences between groups.
Figure 6.
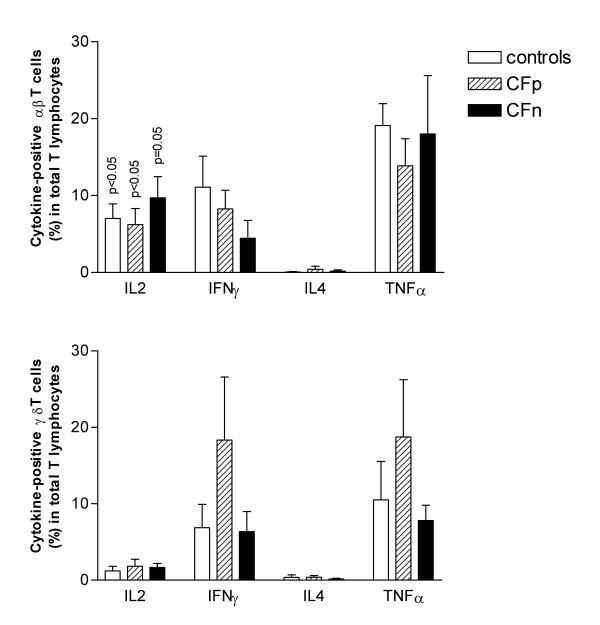
Percentage of intracytoplasmic cytokine-producing αβ T cells (top) and γδ T cells (bottom) within total T lymphocytes after 10 days of culture with cytosolic extract from Pseudomonas aeruginosa, followed by 4 hs restimulation with PMA-Io. Intracytoplasmic cytokine expression was detected, by three color cytometry, in 10000 gated T lymphocytes. Column values correspond to mean, bar values correspond to standard error of the mean. Significant differences were not found between groups (ANOVA). p values were obtained from means comparison between αβ and γδ T lymphocytes within each group: controls (n = 17), Cystic fibrosis patients infected by PA (CFp, n = 9) and Cystic fibrosis patients not infected by PA (CFn, n = 4) (Student t test for paired samples).
Correlation with clinical status
No differences were found in cytokine secretion, activation markers and PBMC proliferation in relation to clinical severity of patients (data not shown).
Discussion
The present study is the first, to our knowledge, that determines the "in vitro" intracytoplasmic cytokine response to Pseudomonas aeruginosa (PA) in αβ and γδ T lymphocytes. We first expanded PA-reactive T cells for 10 days and then we restimulated them with PMA-Io. The cytokine-producing frequencies among αβ and γδ T cells were found in this order: TNF-α>IFN-γ>IL-2, for both subsets. Our results indicate that despite recognizing different antigens from PA [9], αβ and γδ T cells have a similar profile of cytokine secretion, but PAc stimulation induce more γδ than αβ IFN-γ and TNF-α-positive cells. αβ and γδ contribution to the percentage of IFN-γ and TNF-α-producing cells in total T lymphocytic population was the same although the αβ subset was always predominant.
Cytokine production by αβ and γδ T lymphocytes on stimulation with mycobacteria preparations and its purified phosphoantigens has been previosly studied. The secretion profile was limited to Th1-type cytokines [24,25,34-36]. One study compared cytokine levels in supernatants of αβ and γδ purified cells cultured with live Mycobacterium tuberculosis [25] and concluded that γδ T cells were more efficient producers of IFN-γ.
These and our present findings reinforce the evidence of an important role for γδ T lymphocytes in the response to bacteria through IFN-γ secretion [21]. Furthermore, an "in vivo" γδ T memory response, already suggested in humans [37] has been recently described in BCG-vaccinated macaques [26]. This opens the way to new immunization strategies.
On the other hand, our findings did not support the hypothesis that there is a bias in CF T lymphocytes function toward a Th2 response [17,23]. There are contradictory reports in this field. Agreement exists about the protective role of the cellular response to PA [15-17] but the kind of cytokines responsible for a good or a deleterious response in CF is still not elucidated. Some authors demonstrate a beneficial role of the inflammatory cytokine IFN-γ [17] and others implicate a defective production of IL-10 in the pulmonary lesions of CF patients [4]. Moss et al [38], found a lower IFN-γ secretion by CD4-positive T cells in CF patients but the stimulus (anti-CD3 plus PMA) and the tested subpopulation differed from ours.
In a previous work [8] we demonstrated a significant increase of peripheral blood γδ T lymphocytes in cystic fibrosis patients. In later work we showed an "in vitro" preferent γδ T cells proliferation in response to small cytoplasmic phosphorilated non-peptidic compounds from PA, in healthy individuals [9]. Our present results show that the percentage of γδ-positive cells in total T lymphocytes increases to the same extent in healthy and CF individuals after PBMC culture with PAc. In both groups the expanded subset of γδ cells, was Vγ9+Vδ2+CD4-CD8-, already described as predominant in peripheral blood [39,40] and reactive to ubiquitous bacterial phosphorilated compounds [41,42]. In contrast, this subset was reported as not responsive to bacterial phosphoantigens in patients with active pulmonary tuberculosis in comparison to normal PPD+ subjects [14].
In the current study we have not found differences between controls and patients in CD25 and CD45RO expression by γδ T cells after stimulation with PA. Before culture, patients presented a higher percentage of γδ CD45RO-positive than controls, probably due to "in vivo" stimulation by PA or other microorganisms.
In contrast, CD25 and CD45RO expression by CF αβ T lymphocytes was lower than in controls. Selective apoptosis could account for this, but we can not exclude a concomitant low activation-induced expression. Proliferative response to PA was also lower in patients. Therefore, patients are more exposed to PA but they could present a lower percentage of αβ T lymphocytes specific to PA.
Intrinsic and PA-derived T cell defects have already been described in CF patients and in mouse strains prone to chronic infection by PA [5,43]. They have been attributed to inhibitory factors produced by accessory cells or by PA. In this study no significant differences were found between PA-positive and PA-negative patients, although the present work has been mainly focused on intracytoplasmic cytokine production. In addition, patients with moderate and slight severity did not present differences in comparison to patients with good or excellent status. Therefore, Tαβ defects from CF individuals could be related to intrinsic characteristics or to inhibitory mediators produced by other cells.
γδ T cells have a crucial role in protecting airway function and integrity [6,7] but they have been also implicated in some pathogenic cytokine disregulation and development of airway inflammation [11]. Their cytokine production in response to bacterial products is tightly regulated and this regulation depends on contact with live bacteria [44]. We postulate that in CF patients the persistent colonization by PA could avoid the IFN-γ and TNF-α switching-off that develops in acute infection, when the amount of live bacteria decreases.
Finally, this study has been done in T subsets from peripheral blood and further studies at pulmonary level will be needed to elucidate if CF epithelial cells are targets of an excessive inflammatory response mediated by γδ T lymphocytes.
Conclusion
We have demonstrated that γδ-positive T cells from peripheral blood are high "in-vitro" producers of IFN-γ and TNF-α cytokines in response to P. aeruginosa. They contribute at the same extent as the predominant αβ subset to the percentage of cytokine-positive cells in total T lymphocyte subpopulation. The only differences found in cystic fibrosis patients have been in the αβ activation markers expression and the PBMC proliferative response.
γδ T cells could play a crucial role in airway defense against P. aeruginosa and their persistent activation could contribute to the excessive lung inflammation in cystic fibrosis patients.
Abbreviations
BFA: Brefeldin-A
CF: Cystic Fibrosis
CFTR: Cystic Fibrosis Transmembrane Conductance Regulator
c.p.m.: counts per minute
FITC: Fluorescein isothiocyanate
Io: Ionomycine
MAbs: Monoclonal antibodies
PA: Pseudomonas aeruginosa
PAc: Heat treated cytosolic fraction from PA
PBMC: Peripheral blood mononuclear cells
PE: Phycoerytrin
PerCP: Peridin-chlorophyl-A-protein
PHA: Phytohemaglutinin
PMA: Phorbol 12-Myristate 13-Acetate
SC: Schwachman score
SI: Stimulation index
Acknowledgments
Acknowledgements
We thank Ms. F. Oliver and Ms. C. Serra, who kindly took blood samples from cystic fibrosis patients. We also thank Dr. X. De Gracia for his collaboration in obtaining patient samples and Dr. M. Bofill for her useful suggestions. This work was financed by a FIS grant (96/1094) (Ministerio de Sanidad y Consumo. Spain).
Contributor Information
Salvador Raga, Email: sraga305v@cv.gva.es.
M Rosa Julià, Email: mrjulia@hsd.es.
Catalina Crespí, Email: ccrespi@hsd.es.
Joan Figuerola, Email: jfiguerola@hsd.es.
Natalia Martínez, Email: nmartinez@hsd.es.
Joan Milà, Email: jmila@hsd.es.
Núria Matamoros, Email: nmatamoros@hsd.es.
References
- Moss RB. Cystic fibrosis pathogenesis, pulmonary infection, and treatment. Clin Infect Dis. 1995;21:839–851. doi: 10.1093/clinids/21.4.839. [DOI] [PubMed] [Google Scholar]
- Gilligan PH. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggino W. Cystic Fibrosis and the salt controversy. Cell. 1999;96:607–610. doi: 10.1016/s0092-8674(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Moss RB, Bocian RC, Hsu YP, Dong YJ, Kemna M, Wei T, Gardner P. Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) Clin Exp Immunol. 1996;106:374–388. doi: 10.1046/j.1365-2249.1996.d01-826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat N, Rivlin J, Iancu TC. Functional immunoregulatory T-cell abnormalities in cystic fibrosis patients. J Clin Immunol. 1989;9:287–295. doi: 10.1007/BF00918660. [DOI] [PubMed] [Google Scholar]
- King D, Hyde D, Jackson KA. Protective response to pulmonary injury requires gamma/delta T lymphocytes. J Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- Born WK, Lahn M, Takeda K, Kanehiro A, O'Brien RL, Gelfand EW. Role of γδ T cells in protecting normal airway function. Respir Res. 2000;1:151–158. doi: 10.1186/rr26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Payarols J, Julià MR, Matamoros N, Roman J. Increase in peripheral blood of gamma-delta T cells in patients with cystic fibrosis. An Esp Pediatr. 1994;44:239–241. [PubMed] [Google Scholar]
- Julià MR, Serra P, Matamoros N, Raga S, Martínez P. Small cytoplasmic antigens from Pseudomonas aeruginosa stimulate gamma-delta T lymphocytes. Scand J Immunol. 1998;48:672–678. doi: 10.1046/j.1365-3083.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma-delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Zuany-Amorim C, Ruffié C, Hilé S, Vargaftig BB, Pereira P, Petrolani M. Requirement for gamma-delta T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Engel AG, Harper MC. Polymiositis mediated by T lymphocytes that express the gamma/delta receptor. N Eng J Med. 1991;324:877–881. doi: 10.1056/NEJM199103283241303. [DOI] [PubMed] [Google Scholar]
- Catalfamo M, Roura-Mir C, Sospedra M, Aparicio P, Costagliola S, Ludgate M, Pujol-Borrell R, Jaraquemada D. Self-reactive cytotoxic gamma/delta T lymphocytes in Grave's disease specifically recognize thyroid epithelial cells. J Immunol. 1996;156:804–811. [PubMed] [Google Scholar]
- Li B, Rossman MD, Imir T, Oner-Eyuboglu AF, Wa Lee Ch, Biancaniello R, Carding SR. Disease-specific changes in gamma/delta T cell repertoire and function in patients with pulmonary tuberculosis. J Immunol. 1996;157:4222–4229. [PubMed] [Google Scholar]
- Johansen HK, Hougen HP, Cryz SJ, Rygaard J, Hoiby N. Vaccination promotes TH1-like inflamation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am J Respir Crit Care Med. 1995;152:1337–1346. doi: 10.1164/ajrccm.152.4.7551392. [DOI] [PubMed] [Google Scholar]
- Markham RB, Powderly WG. Exposure of mice to live Pseudomonas aeruginosa generates protective cell-mediated immunity in the absence of an antibody response. J Immunol. 1988;140:2039–2045. [PubMed] [Google Scholar]
- Johansen HK, Hougen HP, Rygaard J, Hoiby N. Interferon-gamma treatment decreases the inflamatory response in chronic Pseudomonas aeruginosa pneumonia in rats. Clin Exp Immunol. 1996;103:212–218. doi: 10.1046/j.1365-2249.1996.d01-618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley M, Pabst R, Cripps A. An important role for intestinally derived T cells in respiratory defence. Immunol Today. 1995;16:231–236. doi: 10.1016/0167-5699(95)80165-0. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Emoto M, Hiromatsu K, Yamamoto S, Matsuura K, Gomi H, Ikeda T, Itohara S, Yoshikai Y. The role of gamma/delta T cells in priming macrophages to produce tumor necrosis factor-alfa. Eur J Immunol. 1995;25:1465–1468. doi: 10.1002/eji.1830250551. [DOI] [PubMed] [Google Scholar]
- Takano M, Nishimura H, Kimura Y, Mokuno Y, Washizu J, Itohara S, Nimura Y, Yoshikai Y. Protective roles of gamma/delta T cells and interleukin-15 in Escherichia coli infection in mice. Infect Immun. 1998;66:3270–3278. doi: 10.1128/iai.66.7.3270-3278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human Vγ2Vδ2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI200113584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets. Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Moss RB, Hsu YP, Yssel H. Is CF a Th2 immunoinflammatory disease? Pediatr Pulmonol. 1995;8:289. [Google Scholar]
- Follows GA, Munk ME, Gatrill AJ, Conradt P, Kaufmann SHE. Gamma Interferon and interleukin 2, but not interleukin 4, are detectable in gamma/delta T-cell cultures after activation with bacteria. Infect Immun. 1992;60:1229–1231. doi: 10.1128/iai.60.3.1229-1231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaguchi K, Balaji KN, Boom WH. CD4+ alfa/beta T cell and gamma/delta T cell responses to Mycobacterium tuberculosis. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptative immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso A, Licenziati S, Corulli M, Canaris AD, De Francesco MA, Fiorentini S, Peroni L, Fallacara F, Dima F, Balsari A, Turano A. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry. 1997;27:71–76. doi: 10.1002/(SICI)1097-0320(19970101)27:1<71::AID-CYTO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–711. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Johannisson A, Festin R. Phenotype transition of CD4+ T cells from CD45RA to CD45RO is accompanied by cell activation and proliferation. Cytometry. 1995;19:343–352. doi: 10.1002/cyto.990190409. [DOI] [PubMed] [Google Scholar]
- Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin C. Cytokine flow cytometry: understanding cytokine biology at the single-cell level. J Clin Immunol. 1997;17:195–204. doi: 10.1023/a:1027350226435. [DOI] [PubMed] [Google Scholar]
- Elson LH, Nutman TB, Metcalfe DD, Prussin C. Flow cytometric analysis for cytokine production identifies T helper 1, T helper 2, and T helper 0 cells within the human CD4+CD27- lymphocyte subpopulation. J Immunol. 1995;154:4294–4301. [PubMed] [Google Scholar]
- Miyawaki T, Kasahara Y, Taga K, Yachie A, Taniguchi N. Differential Expression of CD45RO [UCHL1] and its functional relevance in two subpopulations of circulating TCR-gamma/delta+ lymphocytes. J Exp Med. 1990;171:1833–1838. doi: 10.1084/jem.171.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk ME, Gatrill AJ, Kaufmann SHE. Target cell lysis and IL-2 secretion by gamma/delta T lymphocyters after activation with bacteria. J Immunol. 1990;145:2434–2439. [PubMed] [Google Scholar]
- Poccia F, Cipriani B, Vendetti S, Colizzi V, Poquet Y, Battistini L, López-Botet M, Fournié JJ, Gougeon ML. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vgamma9/Vdelta2 T lymphocytes. J Immunol. 1997;159:6009–6017. [PubMed] [Google Scholar]
- Garcia VE, Sieling PA, Gong J, Barnes PF, Uyemura K, Tanaka Y, Bloom BR, Morita CT, Modlin RL. Single-cell cytokine analysis of gamma delta T cell responses to nonpeptide mycobacterial antigens. J Immunol. 1997;159:1328–1335. [PubMed] [Google Scholar]
- Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guérin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;120:518–525. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L, Ciccone E, Mingari MC, Bottino C, Ferrini S, Tambussi G, Melioli G, Grossi CE, Moretta A. Human T lymphocytes expressing γ/δ T cell antigen receptor. Clin Immunol Immunopathol. 1989;50:S117–S123. doi: 10.1016/0090-1229(89)90118-9. [DOI] [PubMed] [Google Scholar]
- Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, Warnke RA, Bhan AK, Strominger JL, Brenner MB. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D, Bender A, Prospero T, Wesselborg S, Janssen O, Pechhold K. The primary response of Human γδ+ T cells to Mycobacterium tuberculosis is restricted to Vγ9-bearing cells. J Exp Med. 1991;173:1331–1338. doi: 10.1084/jem.173.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma/delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Stevenson MM, Kondratieva TK, Apt AS, Tam MF, Skamene E. In vitro and in vivo responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1995;99:98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Das H, Kamath A, Bukowski JF. Human Vγ2Vδ2 T cells produce IFN-γ and TNF-α with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–6201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]


